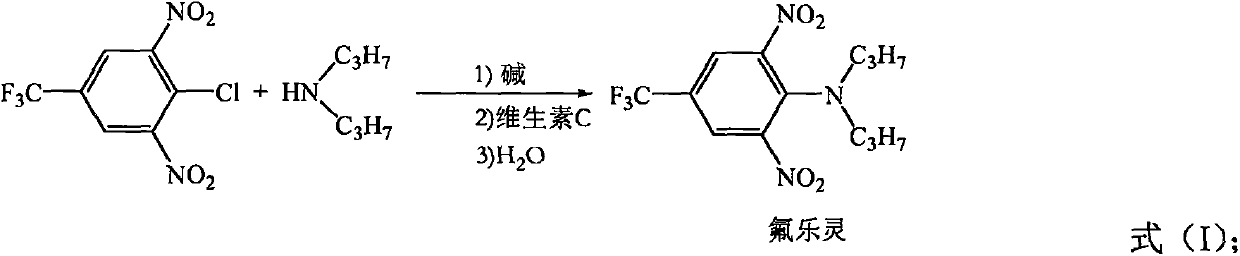
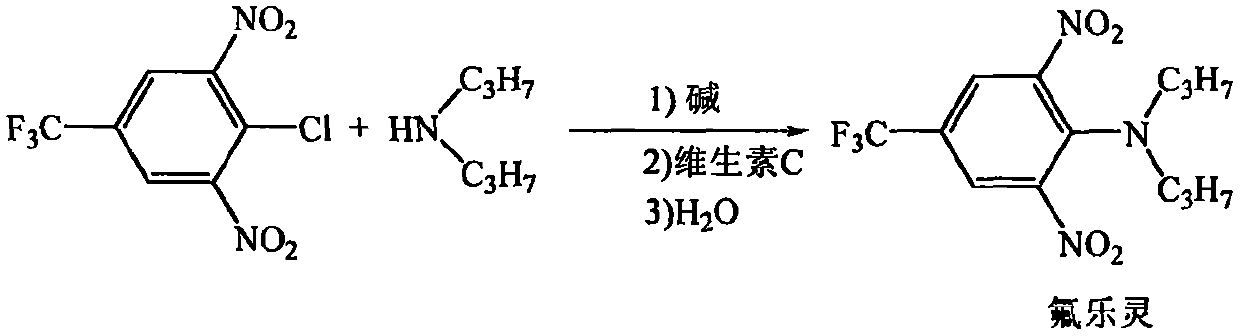
Synthesis method of trifluralin
A synthetic method, trifluralin technology, applied in chemical instruments and methods, amino compound preparation, organic compound preparation, etc., can solve the problems of long synthesis time, environmental hazards, high energy consumption, etc., and achieve low equipment requirements and three wastes The effect of low emission and high conversion rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In a 250ml reactor, add 4-chloro-3,5-dinitrobenzotrifluoride (27.06g, 0.1mol), vitamin C (0.135g), and 30mL water in sequence, and di-n-propylamine (12.14 g, 0.12mol), sodium hydroxide solution (4g of sodium hydroxide dissolved in 16mL of water), the dropwise addition was completed within 30 minutes, and the pH of the system was adjusted to 7.5 to 8.5 with a 1% aqueous sodium hydroxide solution by mass percentage, and the pH of the system was adjusted at 60°C After 2.0 hours of reaction, crystals were precipitated after cooling to room temperature, filtered, washed with water until neutral, and dried to obtain 33.02 g of the target compound with a yield of 98.5% (based on 4-chloro-3,5-dinitrotrifluorotoluene). The product has a purity of 99 wt%, and a nitrosamine content of 0.6 ppm.
Embodiment 2
[0028] In a 250ml reactor, add 4-chloro-3,5-dinitrobenzotrifluoride (27.06g, 0.1mol), vitamin C (0.27g), and 30mL water successively, and di-n-propylamine (10.12 g, 0.1mol), sodium hydroxide solution (4g of sodium hydroxide dissolved in 16mL of water), the dropwise addition was completed within 30 minutes, and the pH of the system was adjusted to 7.5 to 8.5 with 1% by mass percent sodium hydroxide aqueous solution, and the pH of the system was adjusted at 70°C After 1.5 hours of reaction, crystals were precipitated after cooling to room temperature, filtered, washed with water until neutral, and dried to obtain 33.02 g of the target compound with a yield of 98.5% (calculated as 4-chloro-3,5-dinitrotrifluorotoluene). The product has a purity of 99 wt%, and a nitrosamine content of 0.5 ppm.
Embodiment 3
[0030] In a 250ml reactor, add 4-chloro-3,5-dinitrobenzotrifluoride (27.06g, 0.1mol), vitamin C (0.27g), and 30mL water successively, and di-n-propylamine (10.12 g, 0.1mol), sodium hydroxide solution (4g of sodium hydroxide dissolved in 16mL of water), the dropwise addition was completed within 30 minutes, and the pH of the system was adjusted to 7.5 to 8.5 with a 1% aqueous solution of sodium hydroxide by mass percentage, at 80°C The reaction was carried out for 1.0 hour, and crystals were precipitated after cooling to room temperature, filtered, washed with water until neutral, and dried to obtain 33.05 g of the target compound with a yield of 98.6% (calculated as 4-chloro-3,5-dinitrotrifluorotoluene). The product has a purity of 99% by weight and a nitrosamine content of 0.4ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com