Extraction solution for preparing active light calcium carbonate, application and preparation method of active light calcium carbonate
An active light calcium carbonate technology, applied in calcium carbonate/strontium/barium, separation methods, solvent extraction, etc., can solve the problems of low concentration of calcium solution, low purity of calcium carbonate, difficulty in promotion and application of calcium carbonate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
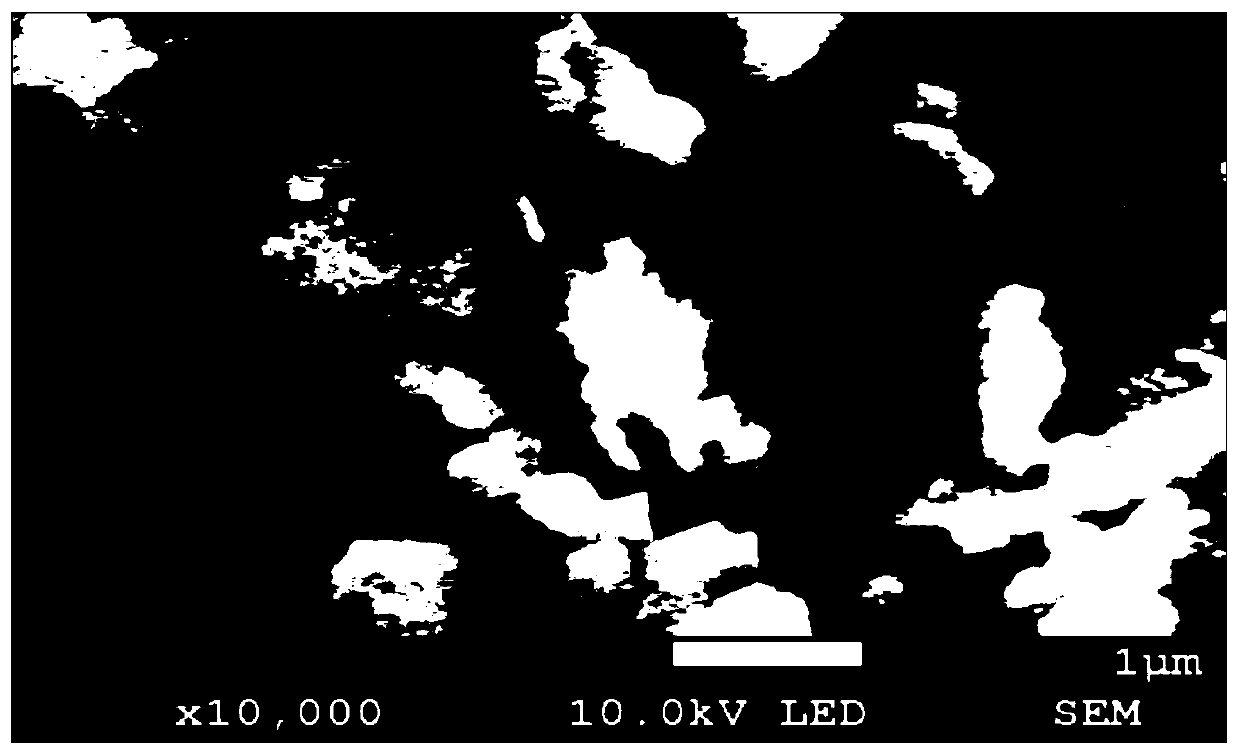
Image
Examples
Embodiment 1
[0115] (1) Take 270g of 4-heptanone, 21g of dibutylamine, and 9g of propionic acid, mix them uniformly, and prepare an organic extraction solution.
[0116] (2) Mix the prepared organic extraction solution with 8% Ca(OH) 2 Mixing calcium carbide slurry to control Ca in calcium carbide slurry 2+ The molar ratio of propionic acid to the organic extract solution is 1:2. After fully reacting, filter out the insoluble matter, stand and separate layers, the upper layer will get an organic solution containing dibutylamine and 4-heptanone, and the lower layer will get an organic solution containing calcium propionate of aqueous solution.
[0117] (3) Put pure CO 2 The gas of step 2) fully contacts with the organic solution that obtains, controls CO 2 with Ca 2+ The molar ratio was 0.9:1, and the organic phase obtained dibutylamine carbonate.
[0118] (4) Add the organic solution containing dibutylamine carbonate that step 2) obtains to the calcium propionate aqueous solution and ...
Embodiment 2
[0121] (1) Get 180g of n-octanol, 72g of dodecylamine, and 15g of formic acid, mix them uniformly, and prepare an organic extraction solution.
[0122] (2) Mix the prepared organic extraction solution with 10% Ca(OH) 2 Mixing calcium carbide slurry to control Ca in calcium carbide slurry 2+ The molar ratio with formic acid in the organic extraction solution is 1:2.2. After fully reacting, filter out the insoluble matter, stand and separate layers, the upper layer will obtain an organic solution containing dodecylamine and n-octanol, and the lower layer will obtain an aqueous solution containing calcium formate.
[0123] (3) Will contain 20wt% CO 2 The flue gas is in full contact with the organic solution obtained in step 2), and the CO is controlled 2 with Ca 2+ The molar ratio is 1:1, and the organic phase obtains dodecylamine carbonate.
[0124] (4) Add the calcium formate aqueous solution obtained in step 2) and the organic solution containing dodecylamine carbonate obt...
Embodiment 3
[0127] (1) Get 50g of butyl acrylate, 172g of octadecylamine, and 25g of acetic acid, mix them uniformly, and prepare an organic extraction solution.
[0128] (2) the prepared organic extraction solution and 20wt% Ca(OH) 2 Lime milk mixed to control Ca in lime milk 2 + The molar ratio with acetic acid in the organic extraction solution is 1:2.3. After fully reacting, filter out the insoluble matter, stand and separate layers, the upper layer will obtain an organic solution containing octadecylamine and butyl acrylate, and the lower layer will obtain an aqueous solution containing calcium acetate.
[0129] (3) Put pure CO 2 The gas of step 2) fully contacts with the organic solution that obtains, controls CO 2 with Ca 2+ The molar ratio is 1.1:1, and the organic phase obtains octadecylamine carbonate.
[0130] (4) Add the calcium acetate aqueous solution obtained in step 2) and the organic solution containing octadecylamine carbonate obtained in step 3) to the reactor and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com