Method of manufacturing bispecific antibodies, bispecific antibodies and therapeutic use of such antibodies
A technology of bispecific antibodies and immunoglobulins, applied in chemical instruments and methods, antibody mimics/scaffolds, anti-animal/human immunoglobulins, etc., can solve the problem of increasing the risk of inducing anti-drug antibodies and failing to mass-produce Issues such as clinical development and commercialization, increasing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
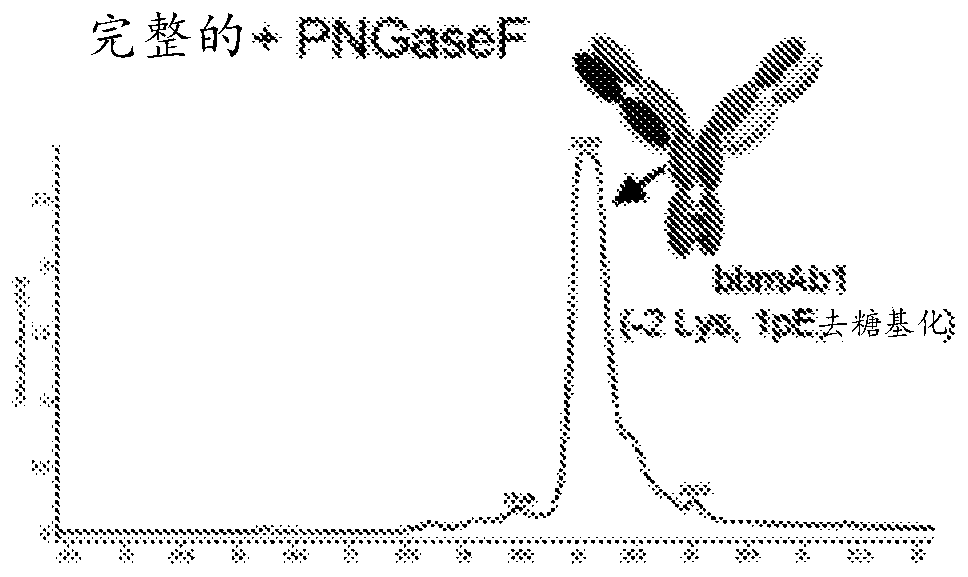
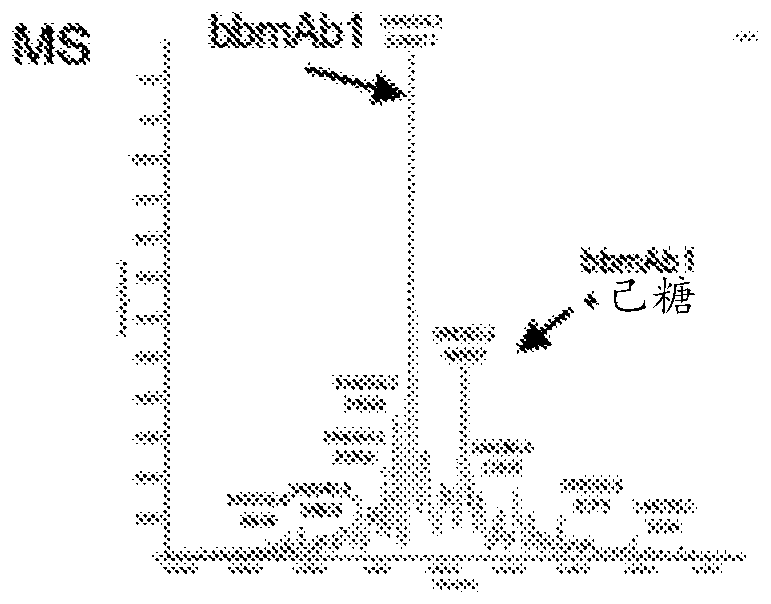
[0192] 5. Example 1: Production of bbmAb bbmAb1
[0193] By way of example, the following describes the generation of specific bbmAbs to enable those skilled in the art to practice the invention.
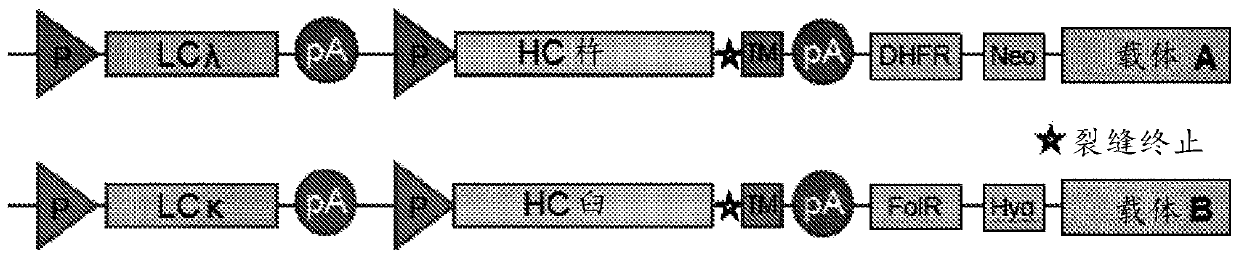
[0194] The resulting bbmAb, bbmAb1, is a bispecific IgG1 with a LALA silent mutation that simultaneously binds to two distinct targets, IL-1β and IL-18. The antibodies bind two distinct antigen-binding arms (Fab fragments), while the Fab against IL-1β is based on mAb2 and contains a kappa light chain (Vk6). The Fab against IL-18 is based on mAb1 and consists of a lambda light chain (Vλ1). To drive heterodimerization of the Fc domain during expression, "knobs" with large amino acid (aa) side chains (S354C and T366W) and small aa side chains (Y349C, T366S, The "holes" of L368A, Y407V) were introduced into the mAb2 heavy chain.
[0195] For ease of reference, the CJM112 hypervariable region based on Kabat definition and Chothia definition, and V L and V H Domain and complete amino...
example 2
[0325] 6. Example 2: In vitro activity of bbmAb1
[0326] The binding activity of bbmAb1 was tested in various cellular assays.
[0327] (1) Materials and methods
[0328] (a) For solution equilibrium titration (SET) determination
[0329] The following materials were used:
[0330] Biotinylated recombinant human IL-18 (BTP25828)
[0331] Recombinant Cynomolgus IL-1β (Novartis)
[0332] SULFO-TAG labeled anti-human IgG antibody (Meso Scale discovery (Meso Scale discovery, MSD) #R32AJ-5)
[0333] Goat anti-human Fab specific antibody conjugated with MSD SULFO-TAG NHS ester (Jackson Immuno Research #109-005-097, MSD #R91AN-1), BSA (Sigma # A-9647)
[0334] MSD Read Buffer T with Surfactant (MSD#R92TC-1)
[0335] Phosphate Buffered Saline (PBS) 10x (Teknova #P0195) Tris Buffered Saline pH 7.5 (TBS) 10x (Teknova #T1680) Tween-20 (Fluka #93773)
[0336] Polypropylene microtiter plate (MTP) (Greiner #781280)
[0337] 384-well plate, standard (MSD#L21XA)
[0338] (b) For ce...
example 3
[0423] 7. Example 3: Effects of combined stimulation and blockade of IL-1β and IL-18 in PBMCs
[0424] Inflammasome activation-dependent cleavage of effector cytokines IL-1β and IL-18 results in the induction of secondary pro-inflammatory mediators that promote immune cell recruitment / activation not only systemically but also at sites of inflammation. In two different mouse models of lethal systemic inflammation (a) LPS injection model and (b) FCAS mice (activating missense mutations in NLRP3), with single IL-1β or single IL-18 absent / inhibited In contrast, simultaneous absence / inhibition of IL-1β and IL-18 was more protective against lethality, demonstrating an additive or synergistic mechanism of immune activation (Brydges 2013, van den Berghe 2014). bbmAb1 is a human / marmoset IL-1β / IL-18 reactive bispecific mAb with no rodent cross-reactivity and therefore could not be tested in mouse models. Therefore, we stimulated human PBMCs using LPS / IL-12 in vitro to mimic activation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com