A kind of crystal form of fatty acid bile acid conjugate and its preparation method and application
A crystal form and compound technology, applied in the field of the crystal form of fatty acid bile acid conjugates and its preparation, can solve the problems of harsh packaging and storage conditions, increased drug production costs, unfavorable production and application, etc., to reduce material storage and quality Control costs, facilitate drug absorption, and avoid the effects of pretreatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0127] Weigh a certain amount of compound (I) solid in a glass vial, add a certain volume of solvent, as shown in Table 1A, make a suspension, stir at room temperature, centrifuge, dry, collect the solid, and mark the obtained solid as Samples 1-a, 1-b, 1-c.
[0128] Table 1A
[0129]
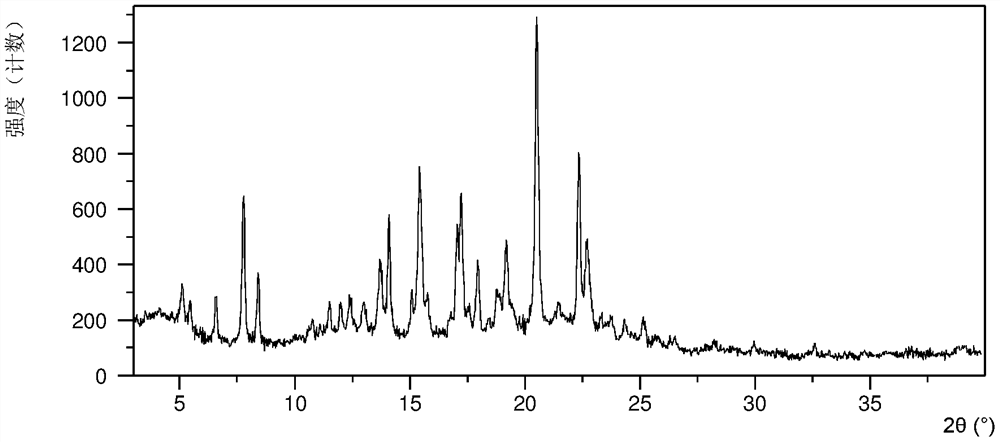
[0130] After testing, samples 1-a, 1-b, and 1-c were all crystal forms CS3. Wherein the XRPD data of sample 1-a are as Figure 1A , as shown in Table 1B, samples 1-b, 1-c have the same or similar XRPD pattern as sample 1-a.
[0131] Table 1B
[0132]
[0133]
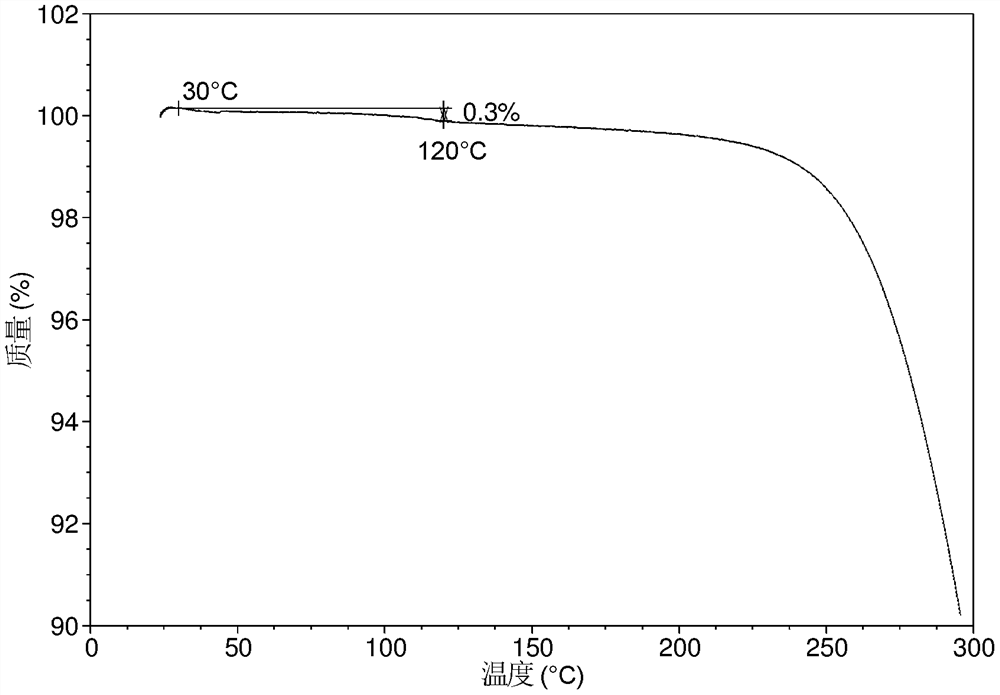
[0134] The TGA of crystal form CS3 is as Figure 1B As shown, it has a mass loss gradient of about 0.3% when heated to 120 °C.
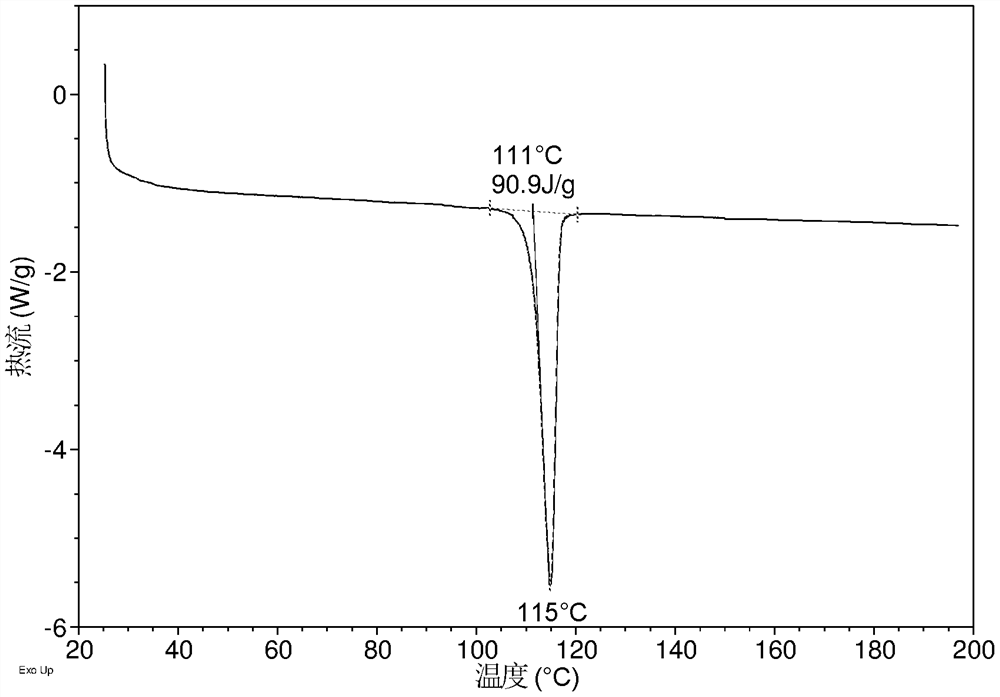
[0135] DSC of crystal form CS3 such as Figure 1C As shown, it has an endothermic peak, and the endothermic peak begins to appear around 111 °C.
[0136] NMR of crystal form CS3 Figure 1D As shown, the NMR data are: 1 HNMR (400MHz, DMSO) δ11.95(s, 1H), 7.53(d, J=7.0Hz, 1H), 4.12(d, J=3.3Hz, 1...
preparation example 2
[0138] Weigh 15.6 mg of compound (I) as a solid, dissolve it in 0.5 mL of chloroform, filter to obtain the filtrate, add 2.0 mL of toluene dropwise to the above 0.2 mL of filtrate, stir until a solid appears, collect the solid by centrifugation, and dry to obtain sample 1- d, after detection, sample 1-d is crystal form CS3.
preparation example 3
[0140] Weigh a certain mass of compound (I) solid, dissolve it in a certain volume of positive solvent in Table 1C, filter to obtain the filtrate, add a certain volume of filtrate to the anti-solvent in Table 1C, if there is solid precipitation, continue to stir until there is A large number of solids were precipitated, and the solids were collected by centrifugation and dried to obtain samples 1-e and 1-f; after testing, samples 1-e and 1-f were all crystal forms CS3.
[0141] Table 1C
[0142]
[0143]
[0144] Crystal form CS3 stability study:
[0145] As the most critical active ingredient in a drug, it is very important for the crystal form to have good physical and chemical stability. The crystal form has good physical stability, which ensures that the raw material drug is not easily transformed into other crystal forms during storage and preparation process, thereby ensuring that the quality of the sample is consistent and controllable.
[0146] Take 2 samples o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com