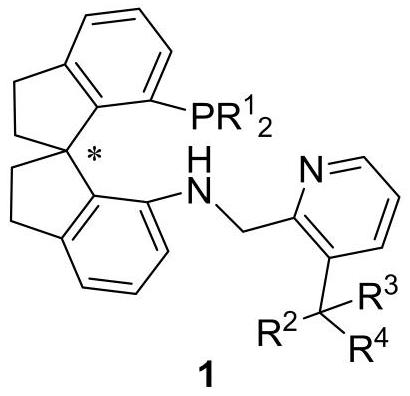
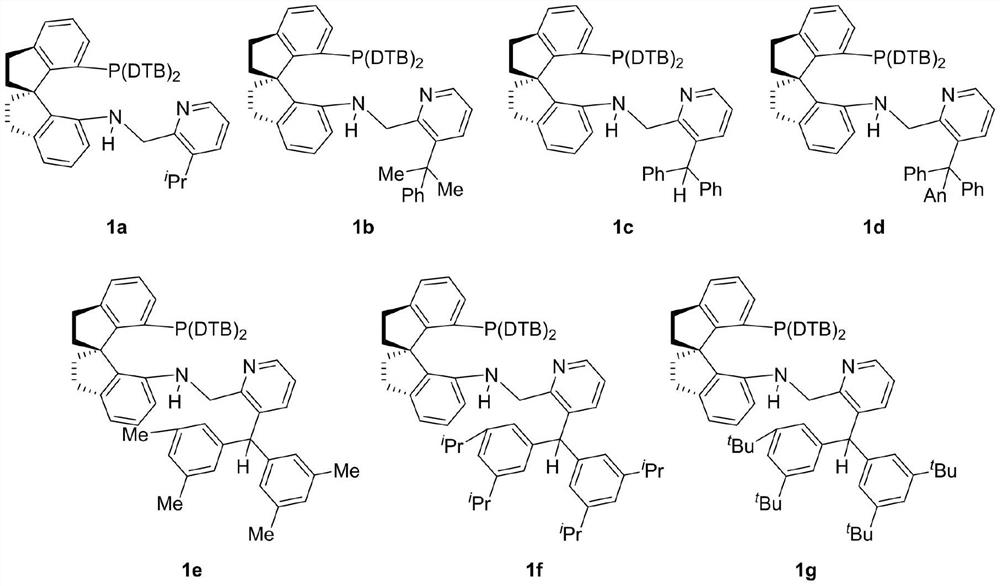
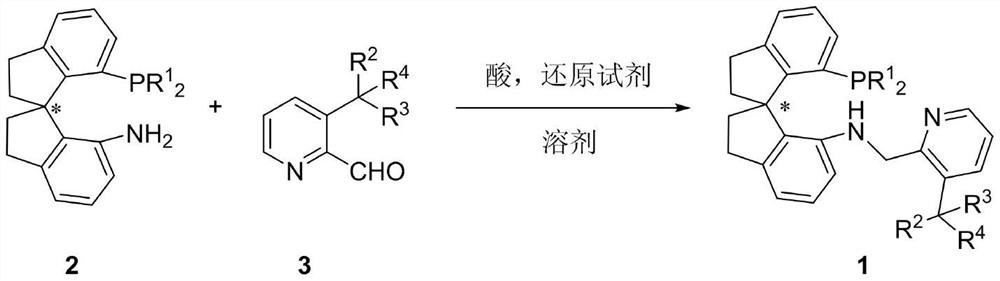
Preparation method and application of chiral spirocyclic aminophosphine ligand substituted at 3-position on pyridine ring
A technology of spiro ring amino group and pyridine ring, which is applied in the field of preparation of chiral spiro ring amino pyridine tridentate ligands, can solve the problems of high catalyst dosage, harsh reaction conditions, narrow substrate range and the like, and achieves a simple synthesis method. , practical synthesis method, the effect of high conversion number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
[0038] A mixture of (R)-DTB-SpiroAP (283 mg, 0.44 mmol) and 3-isopropyl-2-pyridinecarbaldehyde (131 mg, 0.88 mmol) in 1,2-dichloroethane (10 mL) was added under nitrogen atmosphere The reaction was stirred at 45°C for 14h. When the formation of the imine intermediate no longer increased (monitored by TLC), the NaBH(OAc) 3 (148 mg, 0.70 mmol) was added to the system, and the resulting reaction mixture was stirred at the same temperature for 12 h (monitored by TLC). with saturated NaHCO 3 After the solution was quenched, the mixture was extracted with ethyl acetate, the organic phases were combined, the organic phases were dried over anhydrous magnesium sulfate, the drying agent was removed by suction filtration, and the filtrate was removed from the solvent by a rotary evaporator. The residue was purified by silica gel column chromatography (petroleum ether:ethyl acetate=20:1) to obtain 0.22 g of the corresponding colorless syrup with a yield of 97%; –265 (c=0.5...
Embodiment 2
[0040]
[0041] The operation process is the same as that of Example 1a. White solid, mp 74-75°C, 0.35 g, 94% yield. 1 H NMR (400MHz, CDCl 3 )δ: 7.83 (dd, J=4.6, 1.4Hz, 1H), 7.75 (dd, J=8.0, 1.4Hz, 1H), 7.2–7.21 (m, 5H), 7.18–7.07 (m, 5H), 7.07 –6.99(m,2H),6.83(dd,J=7.9,1.8Hz,2H),6.68(dd,J=7.4,1.8Hz,2H),6.55(d,J=7.4Hz,1H),5.74( d, J=7.8Hz, 1H), 5.24 (d, J=7.4Hz, 1H), 3.81–3.58 (m, 2H), 2.97–2.86 (m, 2H), 2.84–2.74 (m, 1H), 2.56 –2.50(m,1H),2.36–2.28(m,1H),1.96–1.88(m,2H),1.72–1.66(m,1H),1.60(s,6H),1.16(s,18H),0.97 (s, 18H); 31 P NMR (162MHz, CDCl 3 )δ:-16.26(s); 13 C NMR (101MHz, CDCl 3 )δ: 156.0, 152.1, 151.9, 149.9, 149.8, 149.4, 149.3, 149.2, 146.0, 145.2(2), 144.2(2), 144.0(2), 141.6, 137.5, 137.4, 137.2, 137.1, 135.2, 135.0 133.2,133.0,131.7(2),129.0,128.8,128.6,128.3,128.0,127.8,126.3,126.2,125.7,125.2,121.7,121.1,120.9,112.7,107.4,71.4,6,2.6,46.8,4 ,35.6,34.8,34.6,31.4,31.3(2),31.1,30.8,30.6(2),27.0.HRMS(ESI)calcd for C 60 H 74 N 2 P[M+H] + :853.5584...
Embodiment 3
[0043]
[0044] The operation process is the same as that of Example 1a. White solid, mp 86-87°C, 0.33 g, 83% yield. 1 H NMR (400MHz, CDCl 3 )δ: 7.85(d, J=4.8Hz, 1H), 7.27–7.22 (m, 2H), 7.19–7.10 (m, 7H), 7.08–7.01 (m, 3H), 6.98–6.92 (m, 3H) ,6.90–6.84(m,3H),6.78(dd,J=7.8,1.6Hz,2H),6.62(dd,J=7.8,1.6Hz,2H),6.54(d,J=8.0Hz,1H), 6.15(d,J=8.0Hz,1H),5.51(s,1H),5.25(d,J=7.6Hz,1H),4.00(dd,J=15.6,6.0Hz,1H),3.66(d,J = 15.2Hz, 1H), 3.00–2.85 (m, 2H), 2.84–2.74 (m, 1H), 2.58–2.52 (m, 1H), 2.41–2.27 (m, 1H), 1.98–1.93 (m, 2H) ), 1.85–1.72(m, 1H), 1.10(s, 18H), 0.90(s, 18H); 31 P NMR (162MHz, CDCl 3 )δ:-16.33(s); 13 C NMR (101MHz, CDCl 3)δ: 155.1, 152.2, 151.9, 149.9(2), 149.5, 149.4, 146.2, 144.7(2), 144.3, 144.2(2), 142.0, 141.7, 137.6, 137.5, 137.1, 137.0, 136.5, 135.1, 134.9 133.1,132.2(2),129.4,129.2,128.8,128.6,128.3,127.9,127.7,126.8(2),126.5,125.3,121.7,121.2,121.1,113.2,107.7,101.0,71.4,61.7,5 42.6,38.0,36.1,34.8,34.6,31.4,31.3,31.2,30.8.HRMS(ESI)calcd for C 64 H ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com