Organic long-afterglow compound, and preparation method and application thereof
A compound and long afterglow technology, applied in organic chemistry, chemical instruments and methods, medical preparations containing active ingredients, etc., can solve the problems of short imaging time and complicated preparation process, and achieve low biological toxicity, long imaging time, Effect of Long Imaging Time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] One, the preparation method of organic long afterglow compound
[0045]The first step: the general preparation method of 4-substituted ether compounds (1):
[0046]
[0047] Compound 4-substituent phenoxy group element phenols (1mmol) and compound 4-substituent halogen benzene (1~3mmol) were dissolved in N,N-dimethylformamide (10mL) solution, after adding sodium carbonate at 60 Stir at ~150°C for 8-12 hours. The formed compound was extracted with ethyl acetate and washed with saturated brine to obtain 4-substituted ether compound 1.
[0048]
[0049] The second step: the general preparation method of 4-heterofluorenes and phenazine substituent ethers (2):
[0050] Compound 1 (1 mmol), heterofluorene-type and phenazine-type substituent compounds (2-6 mmol) were dissolved in N,N-dimethylformamide (20 mL) solution, and stirred at 80-160° C. for 10-36 hours. The formed precipitate was separated by vacuum filtration, washed with saturated brine, and recrystallized i...
Embodiment 1
[0056] Embodiment 1: Synthesis of organic long afterglow compound 3
[0057] The first step: the general preparation method of 4-fluorophenyl ether (1):
[0058]
[0059] Compound 4-fluorophenol (2mmol) and compound 4-fluoroiodobenzene (6mmol) were dissolved in N,N-dimethylformamide (20mL) solution, added sodium carbonate and stirred at 150°C for 24h. The formed compound was extracted with ethyl acetate and washed with saturated brine to obtain compound 1a.
[0060]
[0061] The second step: the general preparation method of 4-carbazolylphenyl ether (2):
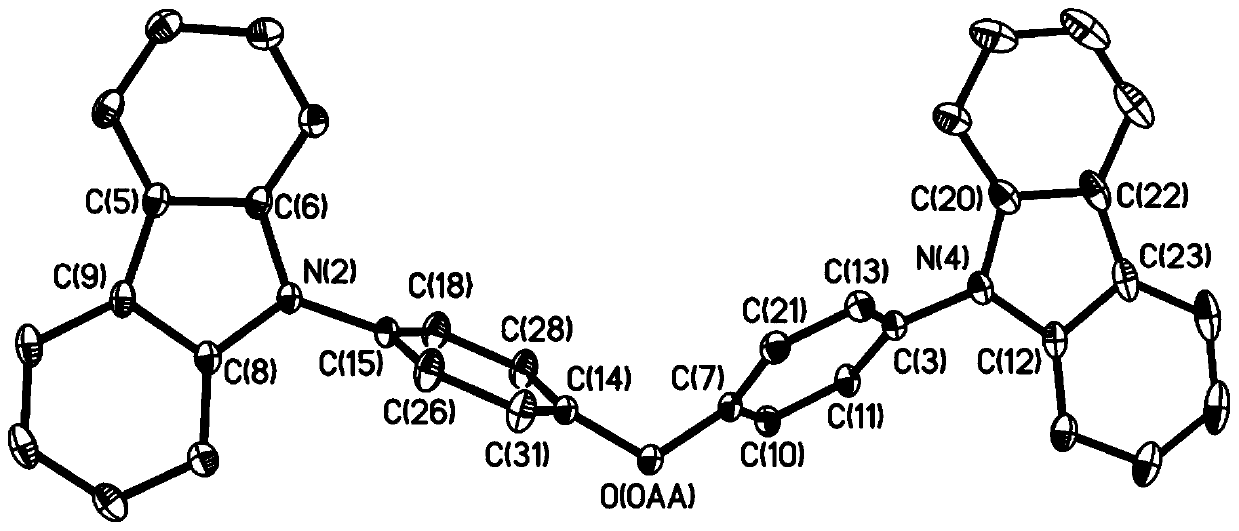
[0062] Compound 1a (2mmol) and carbazole (12mmol) were dissolved in N,N-dimethylformamide (30mL) solution and stirred at 1,60°C for 36h. The formed precipitate was separated by vacuum filtration, washed with saturated brine, and recrystallized in acetone to obtain compound 3, whose structural formula is as follows:
[0063]
[0064] The following are the data of the name, serial number, yield, melting point, colo...
Embodiment 2
[0066] Embodiment 2: Synthesis of organic long afterglow compound 4
[0067] The first step: the general preparation method of 4-fluorophenyl sulfide (1):
[0068]
[0069] Compound 4-fluorothiophenol (2mmol) and compound 4-fluoroiodobenzene (2mmol) were dissolved in N,N-dimethylformamide (20mL) solution, added sodium carbonate and stirred at 60°C for 8 hours. The formed compound was extracted with ethyl acetate and washed with saturated brine to obtain compound 1b.
[0070]
[0071] The second step: the general preparation method of 4-carbazolylphenyl sulfide (2):
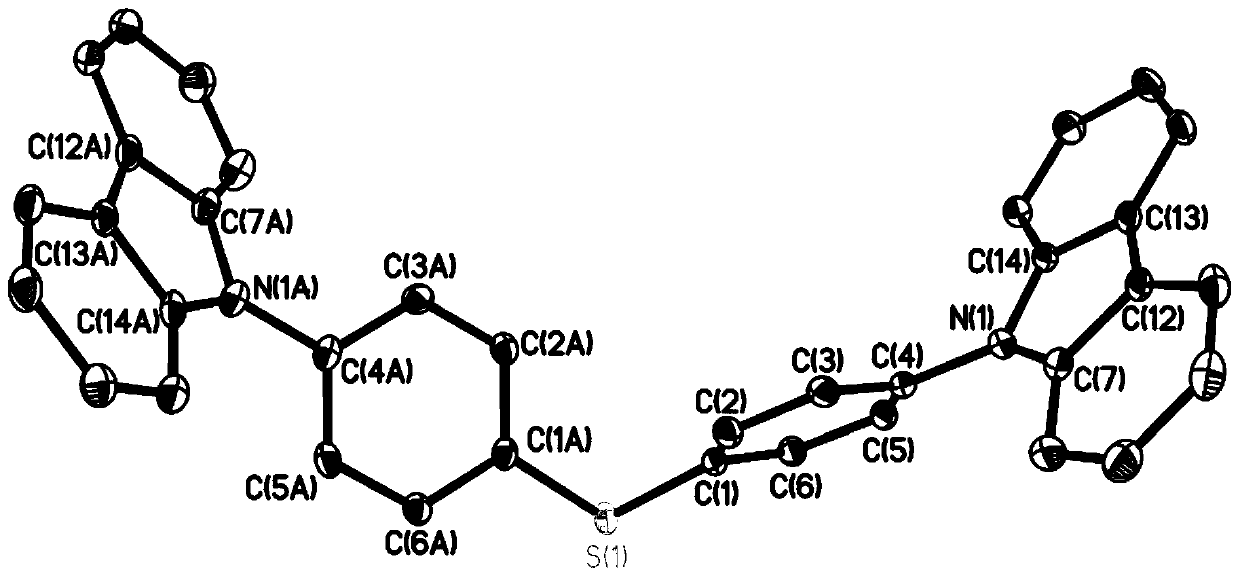
[0072] Compound 1b (2mmol) and carbazole (4mmol) were dissolved in N,N-dimethylformamide (30mL) solution and stirred at 80°C for 10 hours. The formed precipitate was separated by vacuum filtration, washed with saturated brine, and recrystallized in acetone to obtain compound 4.
[0073]
[0074] The following is the name, serial number, yield, melting point, color, state of matter, NMR, mass spectromet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com