Simethicone compound recipe liquid composition and preparation method and application thereof
A technology of liquid composition and simethicone, which is applied in the field of medicine to achieve the effects of good stability, improved clinical curative effect, and enhanced defoaming ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The compound liquid composition of embodiment 1 simethicone and itopride
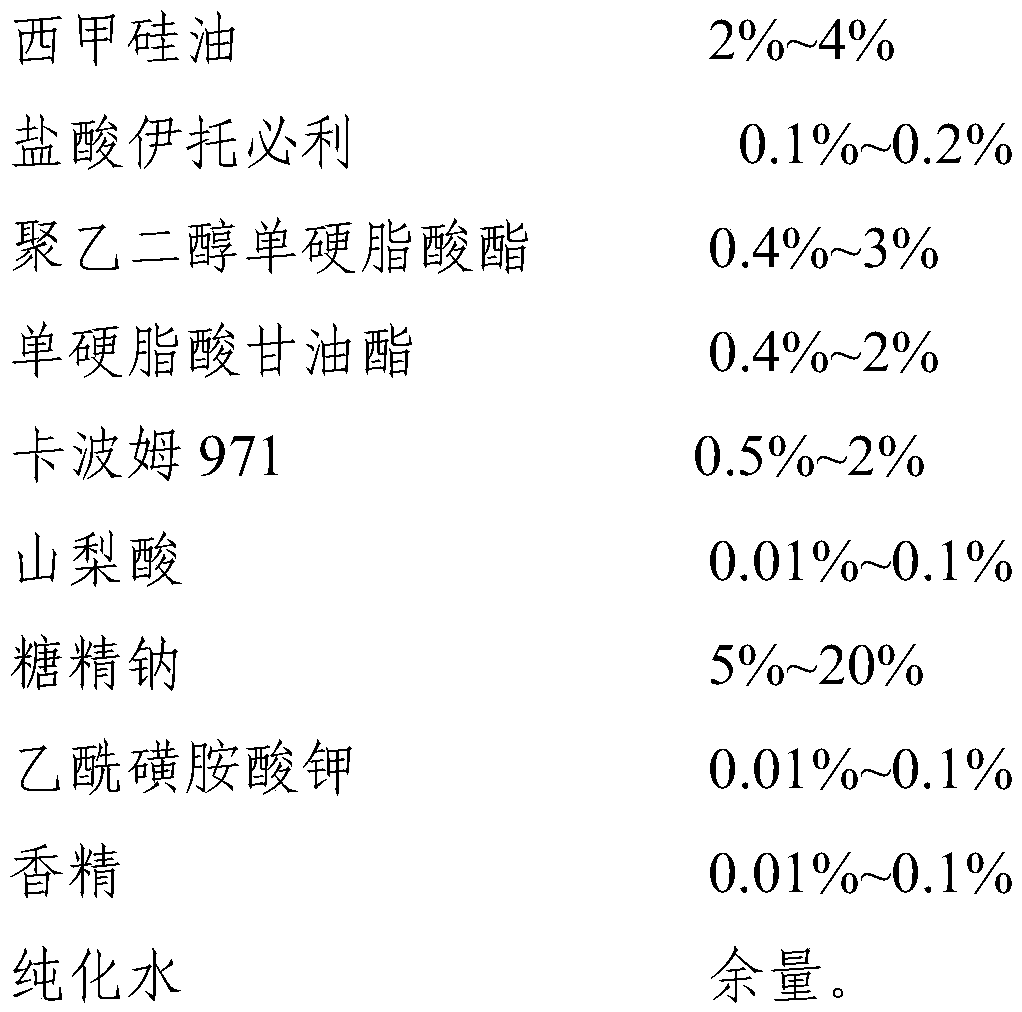
[0038] The proportioning of the compound liquid composition of table 1 simethicone and itopride
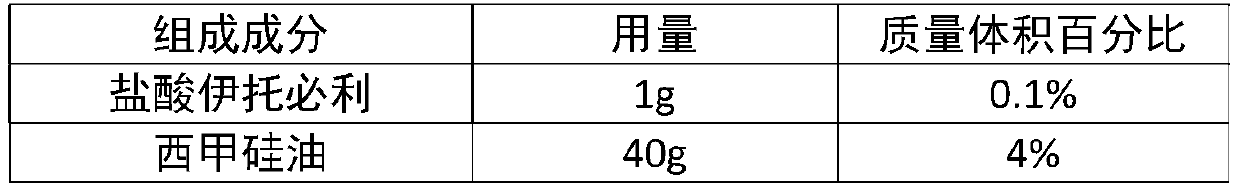
[0039] Composition Dosage mass volume percentage Itopride Hydrochloride 1g 0.1% Simethicone 40g 4% Polyethylene glycol monostearate 30g 3% Glyceryl monostearate 20g 2% Carbomer 971 20g 2% Sorbic acid 0.5g 0.05% sodium saccharin 50g 5% Acesulfame Potassium 0.1g 0.01% banana flavor 0.1g 0.01% sodium hydroxide Appropriate amount Appropriate amount purified water About 830ml 83%
[0040] The present embodiment takes Table 1 as a formula, and prepares pharmaceutical preparations by the following method:
[0041] (1) Stir and mix simethicone and emulsifier at 60-80°C to obtain an oil phase;
[0042] (2) stirring and dissolving itopride hydrochloride, flavoring agent, bacteriostat and 60% purified water of the ...
Embodiment 2
[0045] The compound liquid composition of embodiment 2 simethicone and itopride
[0046] The proportioning of the compound liquid composition of table 2 simethicone and itopride
[0047] Composition Dosage mass volume percentage Itopride Hydrochloride 2g 0.2% Simethicone 20g 2% Tween 40 14g 1.4% Span 40 16g 1.6% Carbomer 974 10g 1% benzoic acid 0.5g 0.02% liquid sorbitol 100g 10% Acesulfame Potassium 0.2g 0.02% orange flavor 0.2g 0.02% sodium hydroxide Appropriate amount Appropriate amount purified water About 840ml 84%
[0048] The preparation method of this embodiment is the same as that of Example 1.
Embodiment 3
[0049] The compound liquid composition of embodiment 3 simethicone and mosapride
[0050] The proportioning of the compound liquid composition of table 3 simethicone and mosapride
[0051] Composition Dosage mass volume percentage Mosapride hydrochloride 0.5g 0.05% Simethicone 5g 0.5% Polyethylene glycol monostearate 6g 0.6% Glyceryl monostearate 4g 0.4% Carbomer Copolymer 20g 2% Potassium sorbate 0.5g 0.05% Sorbitol 80g 8% Acesulfame Potassium 0.1g 0.01% orange flavor 0.1g 0.01% sodium hydroxide Appropriate amount Appropriate amount purified water About 880ml 88%
[0052] In this embodiment, Table 3 is used as a formula, and the pharmaceutical preparation is prepared by the following method:
[0053] (1) Stir and mix simethicone and emulsifier at 65-75°C to obtain an oil phase;
[0054] (2) stirring and dissolving mosapride hydrochloride, flavoring agent, bacteriostat and 70% pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com