A method based on ag NPs to promote the ionization of benzoic acid derivatives
A technology for benzoic acid derivatives, applied in the field of promoting the ionization of benzoic acid derivatives, can solve the problem of few and few control factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] Preferably, the preparation method of Ag NPs includes the following steps: take silver nitrate and deionized water, heat to 130°C under stirring, react at 130°C until the solution boils, and quickly add sodium citrate solution to the reaction system, After reacting for 15-20 minutes, let it stand still and cool down to obtain Ag NPs.
[0047] Preferably, by mass ratio, benzoic acid derivatives:Ag NPs=1:1.
Embodiment 1
[0049] Preparation of Ag NPs
[0050] Put 18 mg of silver nitrate into a two-necked flask, add 100 mL of deionized water, add the rotor, react at 130 ° C until the solution boils, and quickly add 2 mL of 1% sodium citrate solution to the system. It is good to see that the color changes from gray or yellow to mung bean color, then stop the reaction, let it stand until it cools down, and then the Ag NPs solution can be obtained.
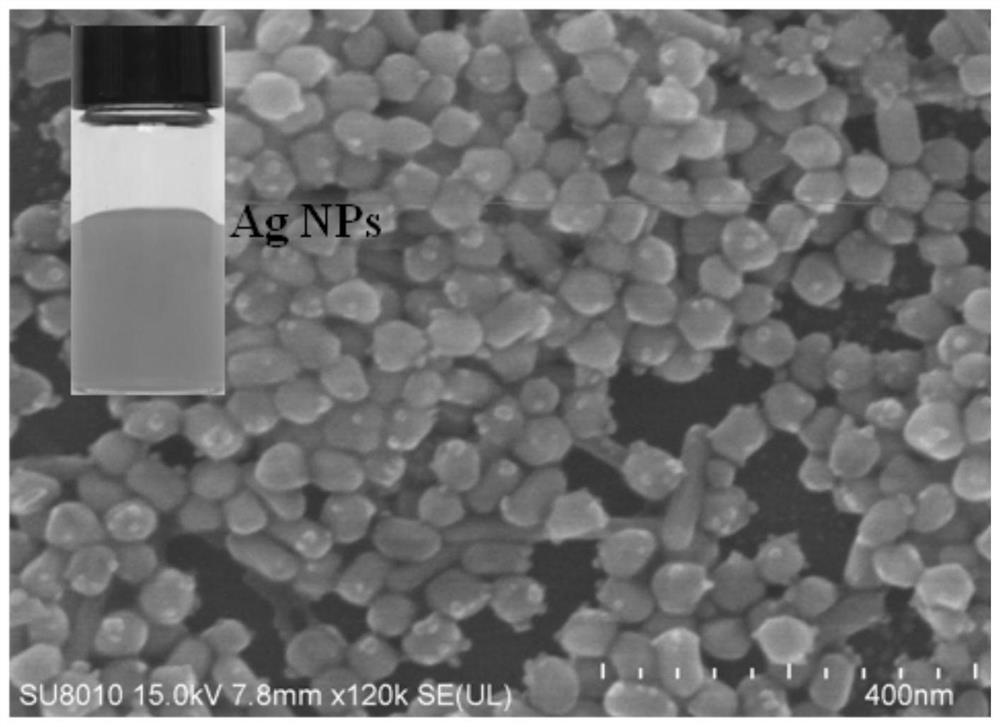
[0051] The prepared Ag NPs solution was centrifuged and washed three times with water, and then a small amount of precipitate was dropped onto a silicon wafer, dried, and tested by SEM. Such as figure 1 As shown, the as-prepared Ag NPs are about 40 nm in size. The inset digital photographs show the form of the product after synthesis. This structure increases the number of "hot spots" for plasmon-driven chemical reactions and the strength of surface plasmon resonance (SPR).
Embodiment 2
[0053] Effects of different amounts of Ag NPs added on the ionization reaction of pMBA
[0054] Methods as below:
[0055] In 3mL the concentration is 10 -5 To the ethanol solution of pMBA of M, add 10-100 μL of the AgNPs solution prepared in Example 1. Then carry out ultraviolet absorption spectrum detection, the result is as follows Figure 2a with Figure 2b .
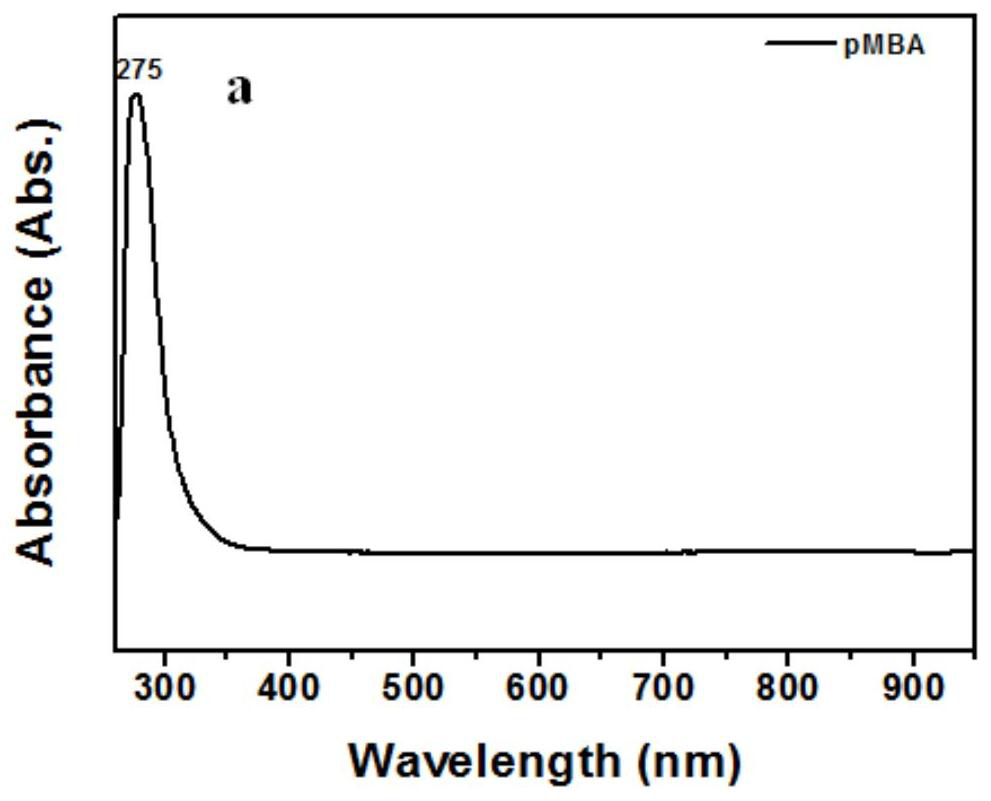
[0056] Figure 2a for 10 -5 M pMBA ethanol solution absorption spectrum, Figure 2b for 10 -5 Absorption spectra of M pMBA mixed with different concentrations of Ag NPs. Depend on Figure 2a Visible, the absorption peak of pMBA ethanol solution is about 275nm, by Figure 2b It can be seen that with the addition of Ag NPs, a new peak appeared around 909nm, indicating the generation of new substances. In order to reflect the irreplaceable role of the hot electrons generated by the Lewis base, different amounts of Ag NPs were added to the ethanol solution of pMBA. It can be clearly seen that the appearance o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com