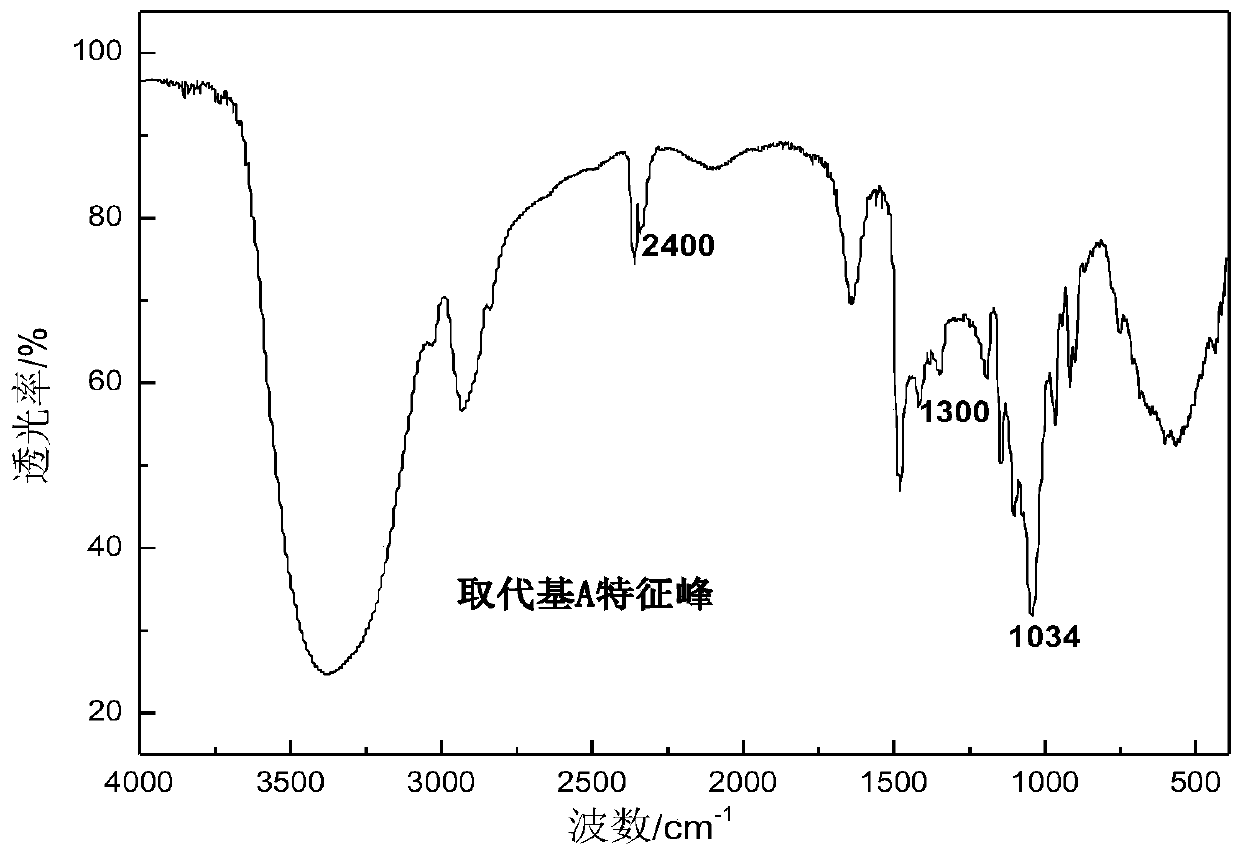
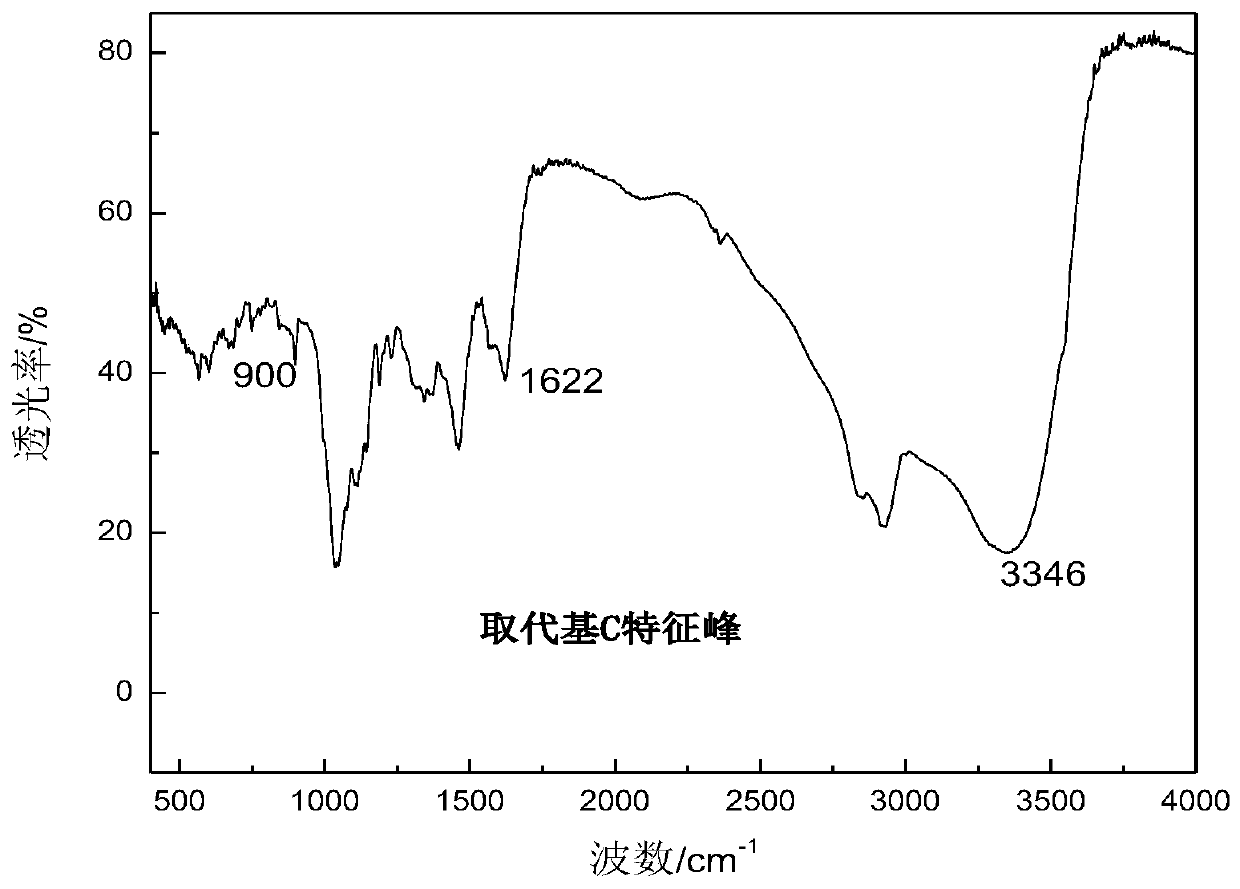
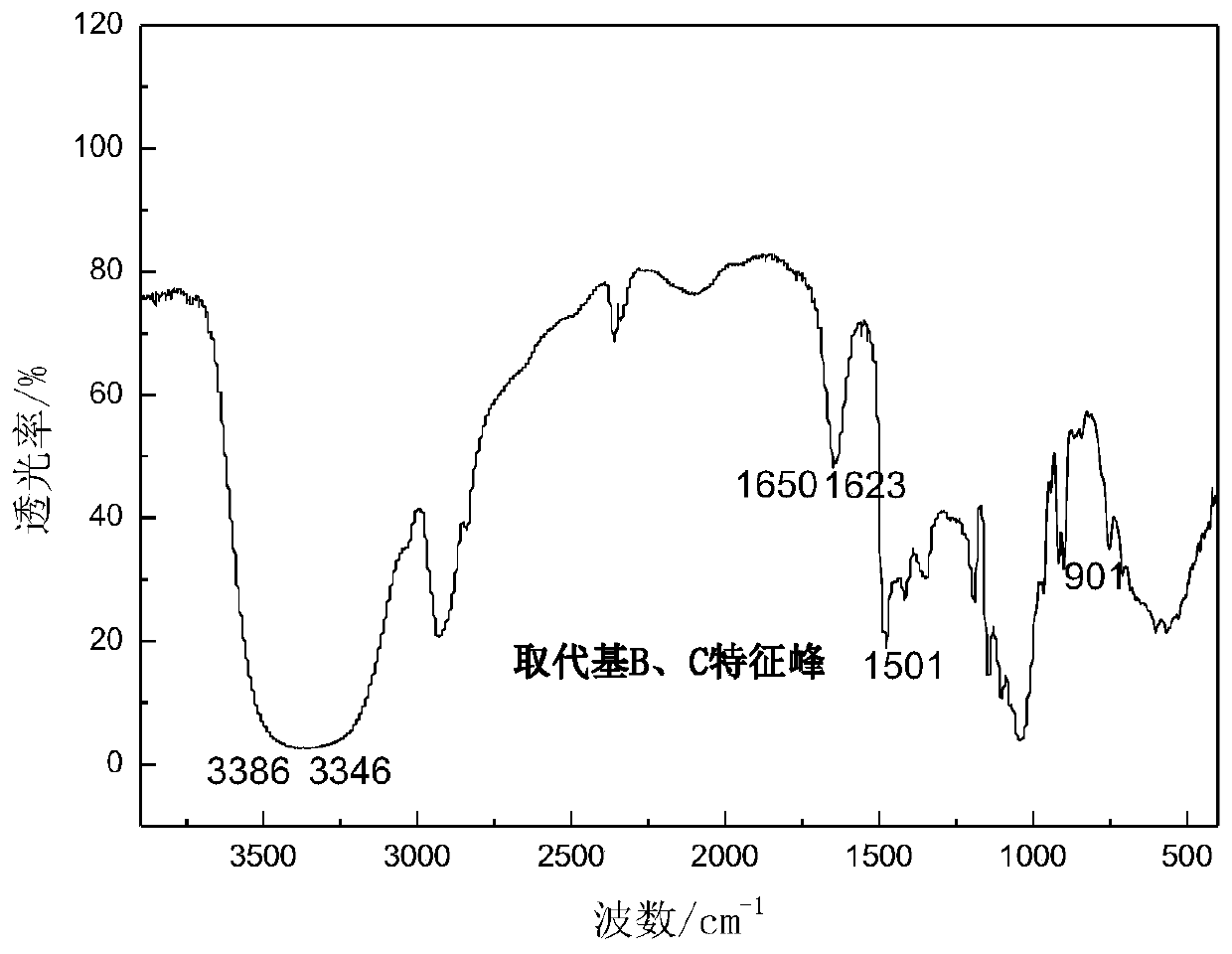
Substituted saccharide or substituted glucoside and application of substituted saccharide or substituted glucoside in drilling fluid composition
A technology of glycosides and substituents, applied in the field of substituted sugars or glycosides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] Add 0.2 mol of epichlorohydrin, 2.4 mol of distilled water and 0.004 mol of p-toluenesulfonic acid into a high-pressure reactor equipped with a thermometer, a condenser, and a stirrer, and react at normal pressure at 60°C for 3 hours to obtain 3-chloro-1 , 2-propanediol aqueous solution;
[0181] Cool the 3-chloro-1,2-propanediol aqueous solution to normal temperature, add 0.4 mol of methyl glucoside to it, and react at normal pressure and 80° C. for 0.5 h to obtain a chlorohydrin glycoside solution;
[0182] The chlorohydrin glycoside solution was neutralized with a saturated aqueous sodium hydroxide solution to a pH value of 7, and then 0.4 moL of a 33.3% trimethylamine aqueous solution was added to the bottom of the chlorohydrin glycoside solution, and the addition was completed within 1 hour. React at 40°C for 3 hours to obtain the substituted glycoside component; the yield of the product is 95.25%. The cationic degree of embodiment 1 product is 0.40mmol / g.
[018...
Embodiment 2
[0187] Add 0.2 mol of epichlorohydrin, 3.2 mol of distilled water and 0.014 mol of p-sulfamic acid into a high-pressure reactor equipped with a thermometer, a condenser, and a stirrer, and react at normal pressure and 80°C for 6 hours to obtain 3-chloro-1 , 2-propanediol aqueous solution;
[0188] Cool the 3-chloro-1,2-propanediol aqueous solution to normal temperature, add 0.22 moL ethyl glucoside to it, and react at normal pressure and 90°C for 3 hours to obtain a chlorohydrin glycoside solution;
[0189] Neutralize the chlorohydrin glycoside solution with saturated potassium hydroxide aqueous solution to a pH value of 7, then add 0.2 moL trimethylamine aqueous solution to the bottom of the chlorohydrin glycoside solution, control the addition within 1 hour, and react at 60°C After 7h, the substituted glycoside component was obtained; the yield of the product was 93.57%. The cationic degree of embodiment 2 product is 1.10mmol / g.
[0190] The substituted glycoside component...
Embodiment 3
[0194] Add 0.2 mol of epichlorohydrin, 3.2 mol of distilled water and 0.014 mol of concentrated sulfuric acid into a high-pressure reactor equipped with a thermometer, condenser, and stirrer, and react at normal pressure at 70°C for 4 hours to obtain 3-chloro-1,2 - aqueous solution of propylene glycol;
[0195] Cool the 3-chloro-1,2-propanediol aqueous solution to normal temperature, add 0.22 moL propyl glucoside to it, and react at normal pressure and 100°C for 4 hours to obtain a chlorohydrin glycoside solution;
[0196] The chlorohydrin glycoside solution was neutralized with saturated sodium carbonate to a pH value of 8, then 0.2 moL tripropylamine was added to the bottom of the chlorohydrin glycoside solution, and the addition was controlled within 1 hour, and the reaction was carried out at 50°C for 4 hours to obtain Substituted glycoside component; the yield of the product is 94.03%. The cationic degree of embodiment 3 product is 1.35mmol / g.
[0197] The substituted g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com