Medicine preparation formulation for treating type 2 diabetes and preparation method of medicine preparation formulation
A type 2 diabetes and drug dosage form technology, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations with non-active ingredients, etc., can solve problems such as insulin deficiency and discounted insulin effects, and achieve stable quality, uniform appearance, and advanced technology Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
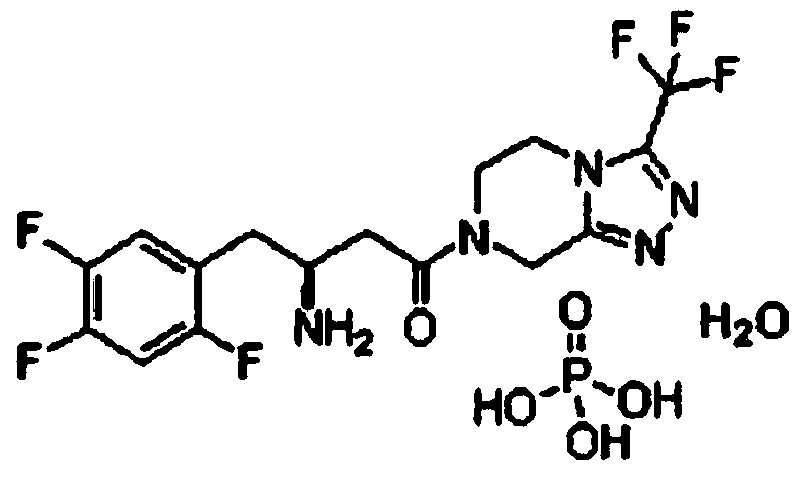
[0035] 1) Preparation of 100mg Sitagliptin Phosphate for Injection
[0036] Prescription composition (1000 prescriptions)
[0037]
[0038] 2) Preparation process:
[0039] (1) Take 80% of the prescribed amount of phosphate buffer solution with a pH value of 4.5 and cool it down to 25°C ± 5°C;
[0040] (2) add dexpanthenol, mannitol, Tween 80 of prescription quantity and make stirring and dissolving, then slowly and evenly add the sitagliptin phosphate of prescription quantity and make stirring fully dissolve;
[0041] (3) Adjust the pH value to 5.06, add 0.1% (w / v) activated carbon and stir evenly, keep the temperature at 25°C±5°C, and adsorb for 30 minutes;
[0042] (4) Decarburization, add phosphate buffer solution with a pH value of 4.5 to the prescribed amount, filter the filtrate through a two-stage 0.22 micron microporous membrane, and fill it into a brown vial that has been sterilized at >350°C for >5 minutes medium, vacuum freeze-dried after half-corking.
[00...
Embodiment 2
[0049] 1) Preparation of 80mg Sitagliptin Phosphate for Injection
[0050] Prescription composition (1000 prescriptions)
[0051]
[0052] 2) Preparation process:
[0053] (1) Take 80% of the prescribed amount of phosphate buffer solution with a pH value of 4.8 and cool it down to 25°C ± 5°C;
[0054] (2) add dexpanthenol, mannitol, Tween 80 of prescription quantity and make stirring and dissolving, then slowly and evenly add the sitagliptin phosphate of prescription quantity and make stirring fully dissolve;
[0055] (3) Adjust the pH value to 5.09, add 0.2% (w / v) activated carbon and stir evenly, keep the temperature at 25°C±5°C, and adsorb for 30 minutes;
[0056] (4) Decarburization, add phosphate buffer solution with a pH value of 4.8 to the prescribed amount, filter the filtrate through a two-stage 0.2-micron microporous membrane, and fill it into a brown vial that has been sterilized at >350°C for >5 minutes medium, vacuum freeze-dried after half-corking.
[0057...
Embodiment 3
[0063] 1) Preparation of 120mg Sitagliptin Phosphate for Injection
[0064] Prescription composition (1000 prescriptions)
[0065]
[0066] 2) Preparation process:
[0067] (1) Take 80% of the prescribed amount of phosphate buffer solution with a pH value of 5.0 and cool it down to 25°C ± 5°C;
[0068] (2) add dexpanthenol, mannitol, Tween 80 of prescription quantity and make stirring and dissolving, then slowly and evenly add the sitagliptin phosphate of prescription quantity and make stirring fully dissolve;
[0069] (3) Adjust the pH value to 5.12, add 0.15% (w / v) activated carbon and stir evenly, keep the temperature at 25°C±5°C, and adsorb for 30 minutes;
[0070] (4) Decarburization, add phosphate buffer solution with a pH value of 5.0 to the prescribed amount, filter the filtrate through a double-stage 0.21-micron microporous membrane, and fill it into a brown vial that has been sterilized at >350°C for >5 minutes medium, vacuum freeze-dried after half-corking.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com