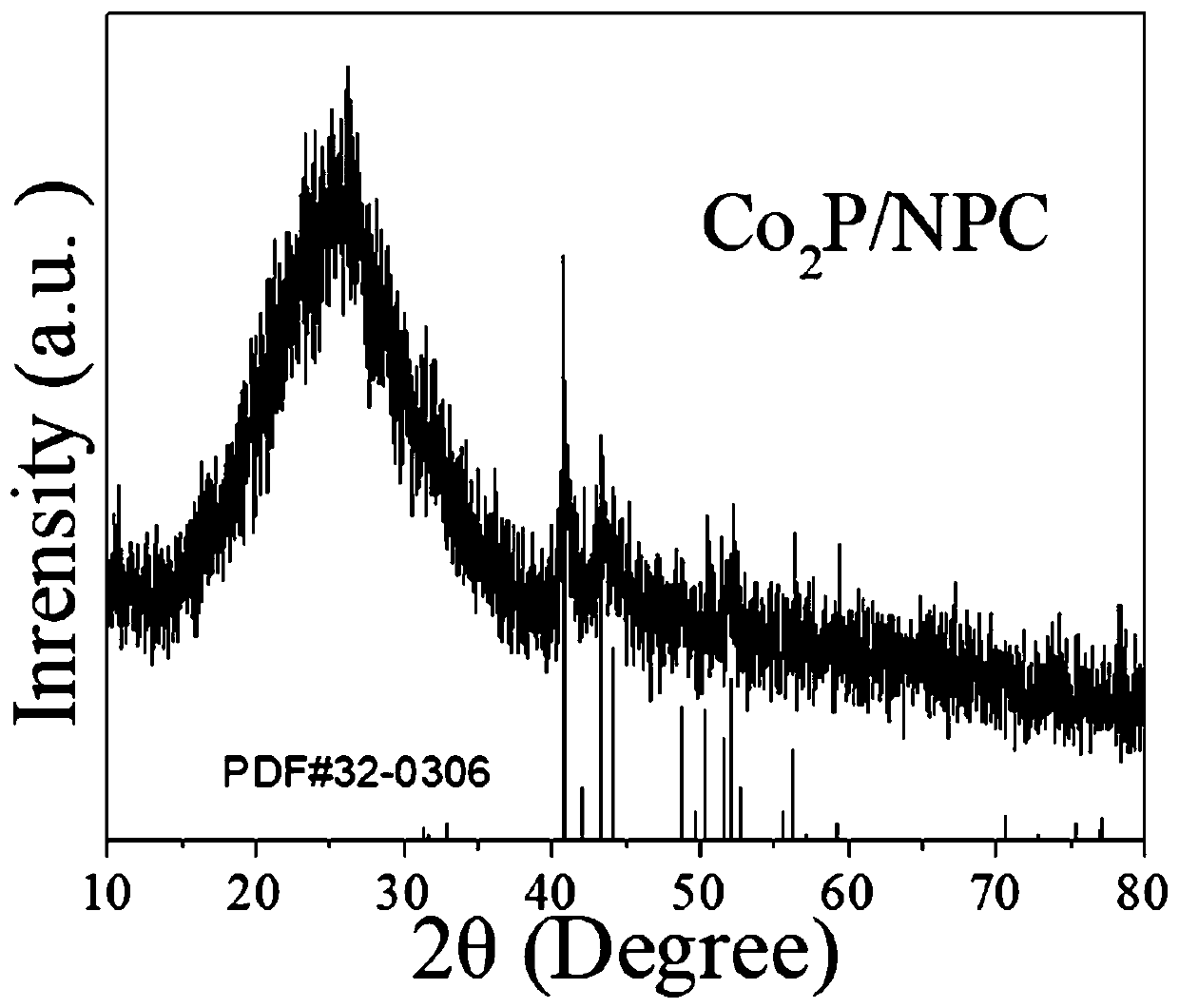
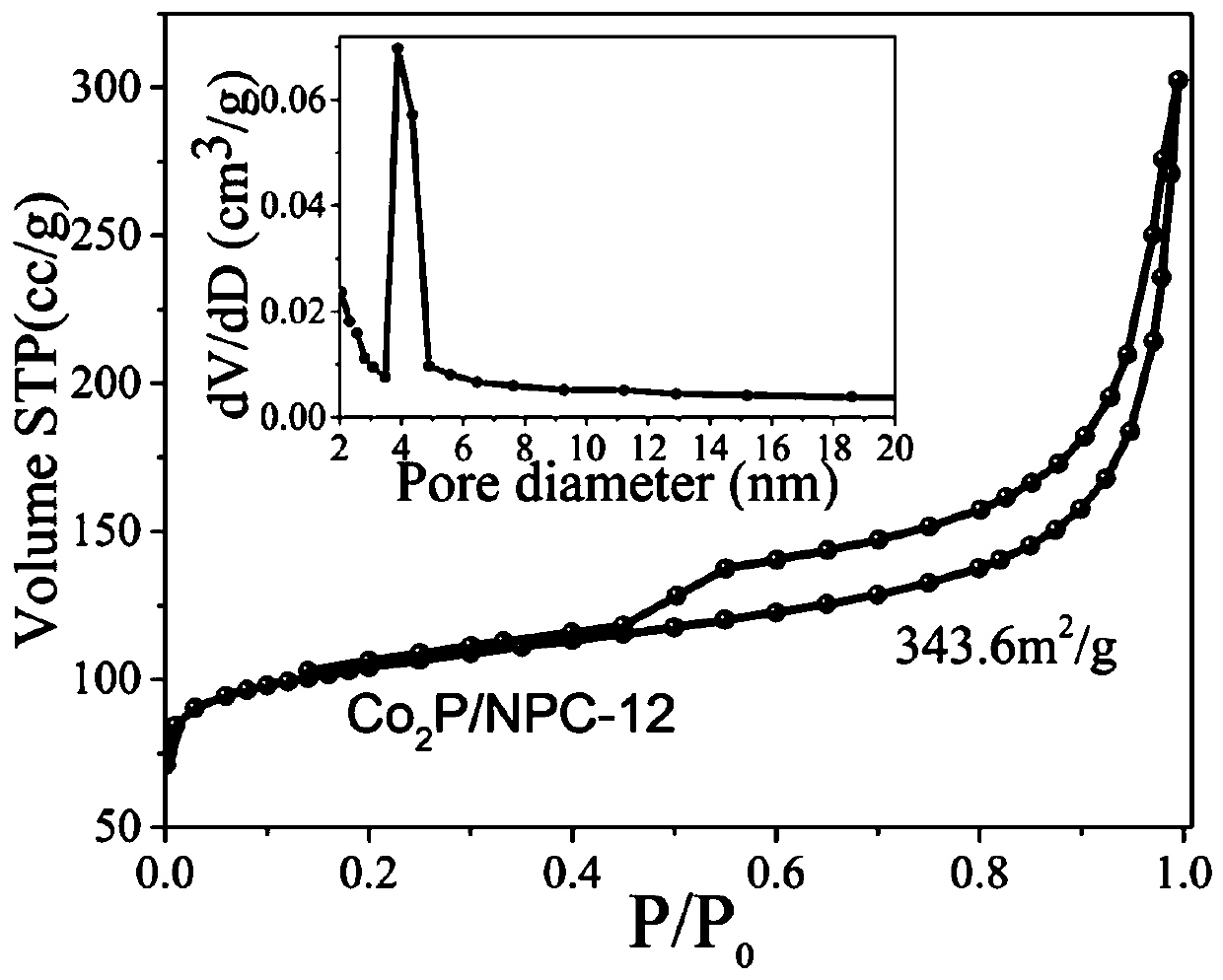
Preparation method of Co2P/NPC electrocatalyst
An electrocatalyst, 2·6H2O technology, applied in circuits, electrical components, battery electrodes, etc., can solve the problems of low electron transfer efficiency, poor bifunctionality, and high OER overpotential, and achieve the effect of improving oxygen reduction performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) Add 0.1gC 14 h 14 N 3 SO 3 Na was added to 120mL ultrapure water, and added in an ice-water bath with stirring
[0019] Add 0.48g FeCl 3 ·6H 2 O, add 0.2mL C 4 h 5 N; add 57.3mg (NPCl 2 ) 3 , kept stirring for 24 hours in the dark under normal temperature conditions, and obtained the precursor polypyrrole after suction filtration, washing and drying;
[0020] (2) Put 60mg polypyrrole in 0.29g Co(NO 3 ) 2 ·6H 2 O ultrapure aqueous solution (10ml)
[0021] Sonicate for 20min, let stand for 12h, centrifuge, wash and dry; place the obtained sample in a place containing 0.082g C 4 h 6 N 2 The ultrapure aqueous solution (10ml) was allowed to stand for 12h, then centrifuged, washed and dried. The obtained sample was placed in the center of the tube furnace for 900 oC High temperature pyrolysis for 1h, to obtain Co 2 P / NPC electrocatalyst. In this step, the annealing atmosphere is high-purity Ar, and the heating rate of the tube furnace is 10 o C / min.
...
Embodiment 2
[0025] (1) Add 0.1gC 14 h 14 N 3 SO 3 Na was added to 120mL of ultrapure water, and 0.48g of FeCl was added in an ice-water bath with stirring 3 ·6H 2 O, add 0.2mL C 4 h 5 N; add 57.3mg (NPCl 2 ) 3, kept stirring for 24 hours in the dark under normal temperature conditions, and obtained the precursor polypyrrole after suction filtration, washing and drying;
[0026] (2) Put 60mg polypyrrole in 0.29g Co(NO 3 ) 2 ·6H 2 Ultrapure aqueous solution (10ml) of O was ultrasonicated for 20min, allowed to stand for 5h, centrifuged, washed and dried; 4 h 6 N 2 The ultrapure aqueous solution (10ml) was allowed to stand for 5h, then centrifuged, washed and dried. The obtained sample was placed in the center of the tube furnace for 900 oC High temperature pyrolysis for 1h, to obtain Co 2 P / NPC electrocatalyst. In this step, the annealing atmosphere is high-purity Ar, and the heating rate of the tube furnace is 10 o C / min.
[0027] Figure 5 For the Co prepared under this...
Embodiment 3
[0029] (1) Add 0.1g C 14 h 14 N 3 SO 3 Na was added to 120mL of ultrapure water, and 0.48g of FeCl was added in an ice-water bath with stirring 3 ·6H 2 O, add 0.2mL C 4 h 5 N; add 57.3mg (NPCl 2 ) 3 , kept stirring in the dark for 24 hours at room temperature, and filtered, washed, and dried to obtain the precursor polypyrrole; put 60 mg of polypyrrole in 0.29 g of Co(NO 3 ) 2 ·6H 2 O ultrapure aqueous solution (10ml) sonicated for 20min, let stand for 2h, centrifuged, washed and dried; the obtained sample was placed in 0.082g C 4 h 6 N 2 Stand in ultrapure aqueous solution (10ml) for 2h, centrifuge, wash and dry. The obtained sample was placed in the center of the tube furnace for 900 oC High temperature pyrolysis for 1h, to obtain Co 2 P / NPC electrocatalyst. In this step, the annealing atmosphere is high-purity Ar, and the heating rate of the tube furnace is 10 o C / min.
[0030] Figure 7 For the Co prepared under this example 2 The ORR stability diagram ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com