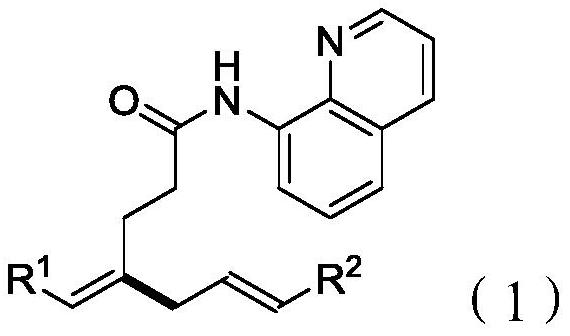
A branched 1,4-dienamide derivative and its synthesis method
A technology of dienamide and synthesis method, applied in the directions of organic chemistry method, organic chemistry, etc., can solve the problems such as no report of the method, and achieve the effects of simple operation, mild reaction conditions and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of (E)-4-benzylidene-N-(quinolin-8-yl)hept-6-enamide
[0043] A screw cap vial was charged with palladium acetate (4.5 mg, 0.02 mmol), dimethylsulfoxide (0.35 mL) and methanol (0.7 mL). Then, pivalic acid (20.4mg, 0.2mmol), (Z)-5-phenyl-N-(quinolin-8-yl)pent-4-enamide (30.2mg, 0.1mmol) and Allyl methyl carbonate (46.4 mg, 0.4 mmol). The vial was sealed under argon and heated to 40°C and stirred for 16 hours. After cooling, the mixture was directly applied to flash column chromatography (PE / EA=4 / 1), and the reaction solution was separated by direct column chromatography to obtain the target product as a white liquid (25.3 mg, yield 74%). The reaction formula is as follows:
[0044]
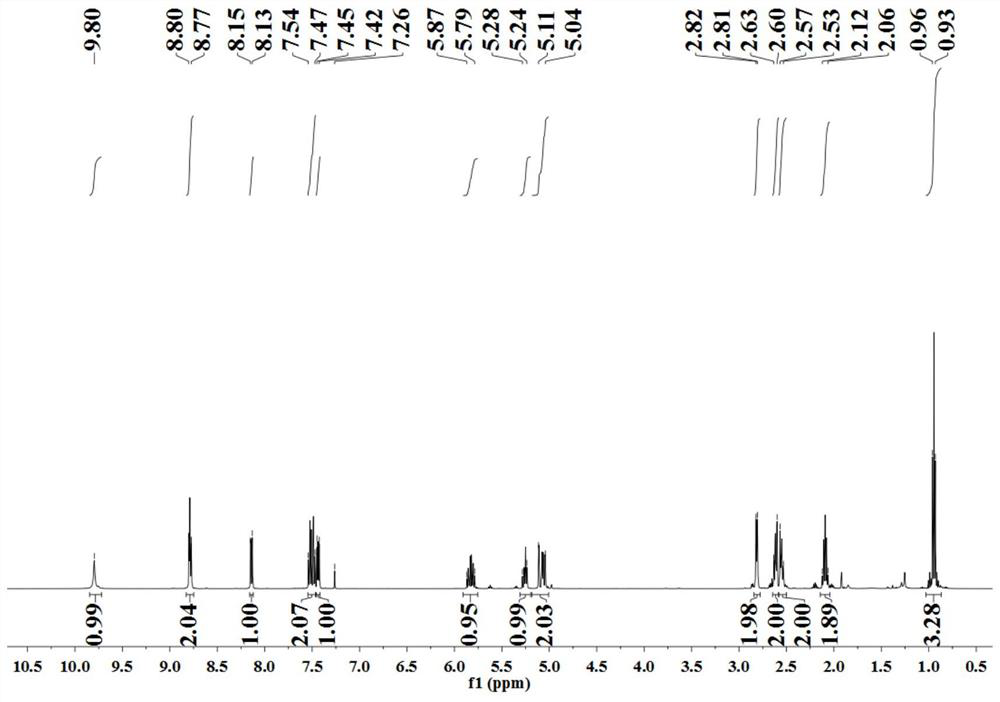
[0045] The nuclear magnetic spectrum characterization result of this reaction product is as follows: 1 H NMR (500MHz, CDCl 3 ): δ9.77(s,1H), 8.77–8.74(m,2H), 8.11(dd,J=8.5,2.0Hz,1H),7.52–7.45(m,2H),7.41(dd,J=8.5 ,4.0Hz,1H),7.30–7.24(m,4H),7.18–7.15(m,1H),6.39(s,1H),5.96–5...
Embodiment 2
[0048] Preparation of (E)-4-(4-fluorobenzylidene)-N-(quinolin-8-yl)hept-6-enamide
[0049] A screw cap vial was charged with palladium acetate (4.5 mg, 0.02 mmol), dimethylsulfoxide (0.35 mL) and methanol (0.7 mL). Then, pivalic acid (20.4 mg, 0.2 mmol), (Z)-5-(4-fluorophenyl)-N-(quinolin-8-yl)pent-4-enamide (32.0 mg , 0.1 mmol) and allyl methcarbonate (46.4 mg, 0.4 mmol). The vial was sealed under argon and heated to 40°C and stirred for 16 hours. After cooling, the mixture was directly applied to flash column chromatography (PE / EA=4 / 1), and the reaction solution was separated by direct column chromatography to obtain the target product yellow liquid (33.4 mg, yield 93%). The reaction formula is as follows:
[0050]
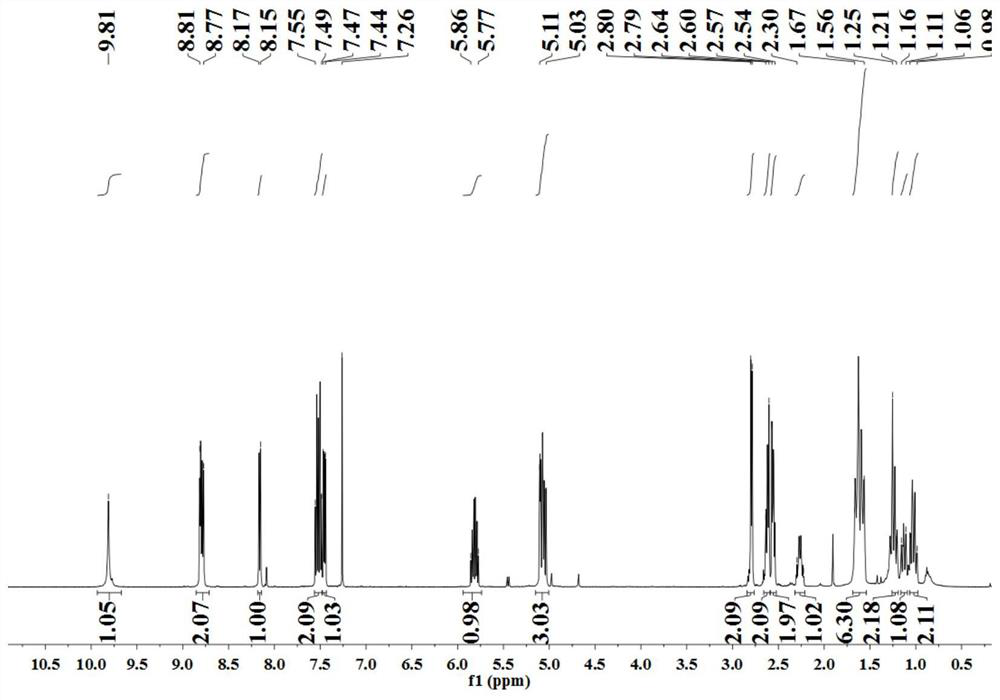
[0051] The nuclear magnetic spectrum characterization result of this reaction product is as follows: 1 H NMR (500MHz, CDCl 3 )δ9.76(s,1H),8.77–8.74(m,2H),8.13(dd,J=8.0,1.5Hz,1H),7.53–7.47(m,2H),7.43(dd,J=8.0, 4.0Hz, 1H), 7.20(q, J=5.5Hz, 2H), 6.96(t, J=9...
Embodiment 3
[0053] Preparation of (E)-4-(4-methoxybenzylidene)-N-(quinolin-8-yl)hept-6-enamide
[0054] A screw cap vial was charged with palladium acetate (4.5 mg, 0.02 mmol), dimethylsulfoxide (0.35 mL) and methanol (0.7 mL). Then, pivalic acid (20.4 mg, 0.2 mmol), (Z)-5-(4-methoxyphenyl)-N-(quinolin-8-yl)pent-4-enamide ( 33.2 mg, 0.1 mmol) and allyl methcarbonate (46.4 mg, 0.4 mmol). The vial was sealed under argon and heated to 40°C and stirred for 16 hours. After cooling, the mixture was directly applied to flash column chromatography (PE / EA=4 / 1). The reaction solution was separated by direct column chromatography to obtain a yellow liquid (28.0mg, yield 76%), the reaction formula is as follows:
[0055]
[0056] The nuclear magnetic spectrum characterization result of this reaction product is as follows:. 1 H NMR (500MHz, CDCl 3)δ9.79(s,1H),8.79–8.75(m,2H),8.15(dd,J=8.5,1.5Hz,1H),7.54–7.48(m,2H),7.44(dd,J=8.5, 4.5Hz, 1H), 7.20(d, J=9.0Hz, 2H), 6.84–6.82(m, 2H), 6.33(s, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com