A kind of polypeptide synthesis method and application thereof containing tyrosine sulfate modification
A technology for peptide synthesis and tyrosine, which is applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of complex process, low yield and high cost, and achieve the effect of broadening the scope.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: Exploration of the conditions for removing the protective group-neopentyl group on the tyrosine sulfate radical:
[0100] Fmoc-Tyr(OSO 3 nP)-OH were dissolved in 100% H 2 O (incomplete dissolution), 90% H 2 O / ACN, 80%H 2 O / ACN, 70%H 2 O / ACN, 60%H 2 O / ACN, 50%H 2 O / ACN, 40%H 2 O / ACN, 30%H 2 O / ACN, 20%H 2 O / ACN, 10%H 2 O / ACN, 100% ACN solution to a concentration of 1mg / ml, room temperature shaking reaction for 2 days, estimated its neopentyl removal rate by the peak area of liquid chromatography mass spectrometry (LC-MS), its removal The rates were 100%, 83%, 75%, 59%, 22%, 18%, 11%, 2%, 4%, 1%, and 0%, respectively. It can be seen that as the water component in the solution increases, the neopentyl removal rate is higher in the same time period.
[0101] Fmoc-Tyr(OSO 3 nP)-OH was dissolved in a small amount of ACN (poor water solubility), and buffered saline solution was added until the acetonitrile ratio was 20%, the concentration was 0.1mg / ml, ...
Embodiment 2
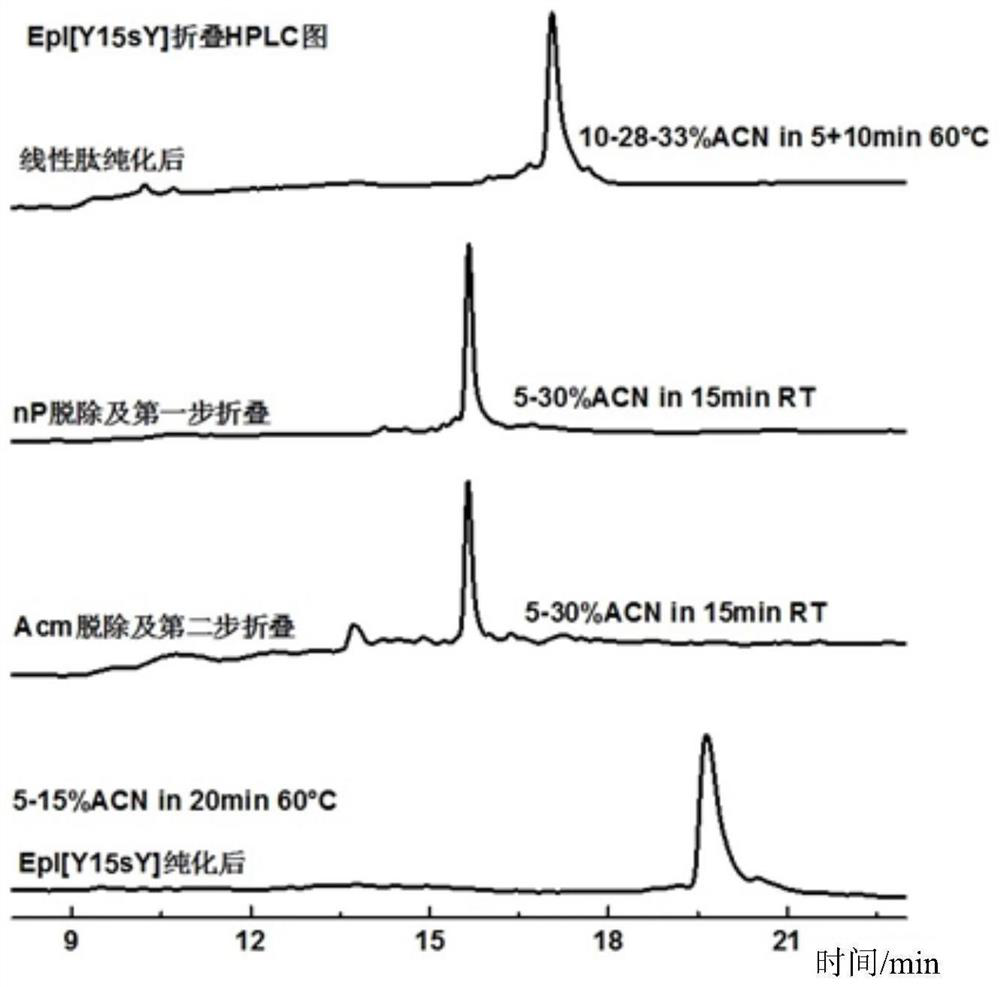
[0103] Embodiment 2: the preparation method of α-conotoxin EpI[Y15sY]:
[0104] (1) Preparation of Fmoc-Cys(Acm)-MBHA resin: Take 0.5g Rink Amide MBHA resin in a peptide synthesis tube with a loading capacity of 0.2-0.8mmol / g, add 10mL DMF to swell twice at room temperature, 15min each time , pumped dry, add 5mL 20% piperidine / DMF to the resin, shake reaction at room temperature for 5min, wash twice with DMF, add 5mL 20%piperidine / DMF again, shake reaction at room temperature for 5min, wash with DMF, DCM in turn , DMF were washed twice each, and the solvent was drained to obtain the resin obtained by removing the Fmoc protection from the amino group. Fmoc-Cys (Acm)-OH (0.3mmol), HOBT (0.6mmol) and DIC (0.3mmol) were weighed, Fmoc- Dissolve Cys(Acm)-OH and HOBT with a small amount of DMF, add DIC, and activate the carboxyl group by shaking at room temperature for 20 minutes, then add the activated amino acid to the resin, shake and react at room temperature for 2 hours, wash wi...
Embodiment 3
[0108] Embodiment 3: the preparation method of α-conotoxin AnIA[Y14sY]:
[0109] (1) Preparation of Fmoc-Cys(Acm)-MBHA resin: According to the method of Example 2, a dry resin with a loading capacity of 0.6g0.5mmol / g was obtained.
[0110](2) Preparation of AnIA[Y16sY]-MBHA resin: Add 0.6g of the obtained Fmoc-Cys(Acm)-MBHA resin to 10mL DMF and swell twice at room temperature, 15min each time, drain, and then add 5mL 20 % acetic anhydride / DMF, shake at room temperature for 20 minutes to block the amino group of the resin that is not coupled with amino acid, prevent its next reaction, wash twice with DMF, DCM, DMF in turn, add 5mL 20% piperidine / DMF to the resin , shaken at room temperature for 5 minutes, washed twice with DMF, added 5 mL of 20% piperidine / DMF again, shaken for 5 minutes at room temperature, washed twice with DMF, DCM, DMF in turn, drained the solvent, and obtained Fmoc-protected resin, weigh Fmoc-L-Tyr (OSO 3 nP)-OH (0.6mmol), HOBT (1.2mmol) and DIC (0.6mmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com