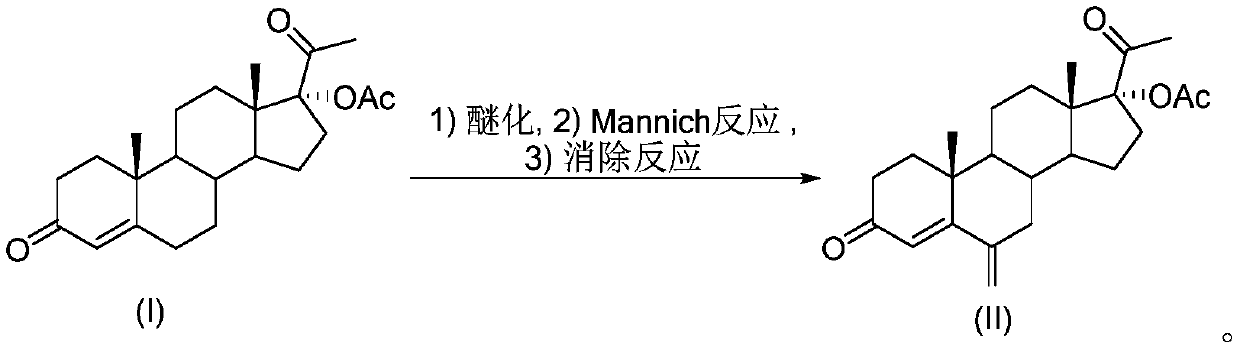
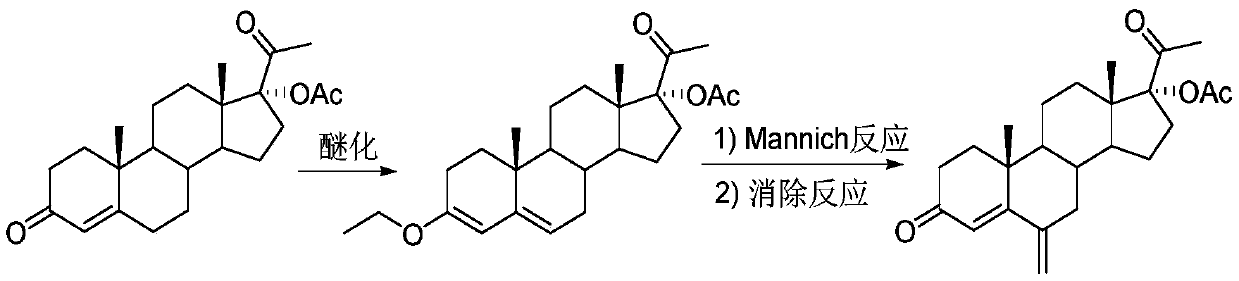
17 alpha-acetoxy-6-methylenepregn-4-ene-3,20-dione preparation method
A technology of methylene pregnan and acetoxy is applied in the field of medicine and chemical industry, which can solve the problems of poor reaction selectivity, large amount of waste liquid, large amount of material, etc., and achieves reduction of side reactions, small reaction volume, and continuous production process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
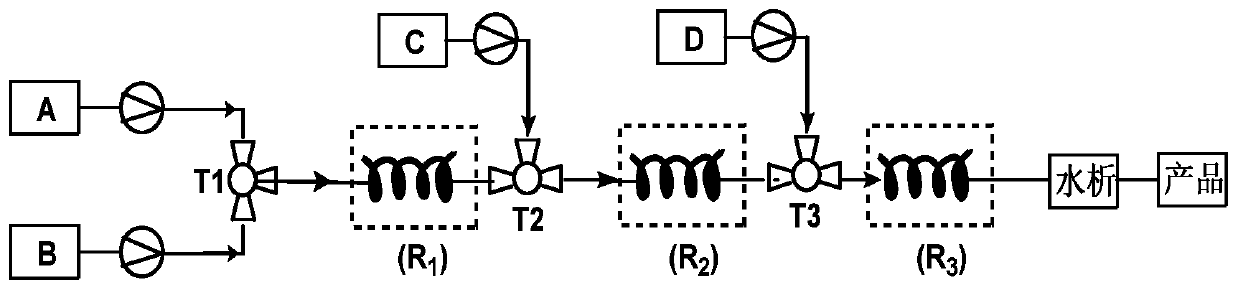
[0026] according to figure 1 In the process flow shown, 17α-hydroxyprogesterone acetate (100mmol, 37.2g), p-toluenesulfonic acid (2mmol, 0.34g) and 300mL tetrahydrofuran were formulated into solution A and placed in a 1L single-port reaction flask; Ethyl ester (120mmol, 17.8g) was dissolved in 100mL ethanol to form solution B, and placed in a 500mL single-necked flask. The materials of solution A and solution B are transported through the metering pump respectively, enter the first mixer for mixing, and adjust the flow through the metering pump to make the 17α-hydroxyprogesterone acetate, p-toluenesulfonic acid and triethyl orthoformate in the mixer The molar flow ratio of the ester is 1:0.02:1.2. The mixed solution in the first mixer enters the first tubular reactor for etherification reaction at 25°C. After staying in the tubular reactor for 0.5h, the ether The reacted material flows out from the first tubular reactor.
[0027] Mix N-methylaniline (120mmol, 12.9g) with 9mL...
Embodiment 2
[0033] according to figure 1 In the process flow shown, 17α-hydroxyprogesterone acetate (100mmol, 37.2g), p-toluenesulfonic acid (5mmol, 0.86g) and 350mL tetrahydrofuran were formulated into solution A and placed in a 1L single-port reaction flask; Ethyl ester (150mmol, 22.2g) was dissolved in 120mL ethanol to form solution B, and placed in a 500mL single-necked flask. The materials of solution A and solution B are transported through the metering pump respectively, enter the first mixer for mixing, and adjust the flow through the metering pump to make the 17α-hydroxyprogesterone acetate, p-toluenesulfonic acid and triethyl orthoformate in the mixer The molar flow ratio of the esters is 1:0.05:1.5, and the mixed liquid in the first mixer enters the first tubular reactor for etherification reaction at 30°C, stays in the tubular reactor for 45 minutes, and etherifies The reacted material flows out from the first tubular reactor.
[0034] Mix N-methylaniline (100mmol, 10.7g) wi...
Embodiment 3
[0037] according to figure 1 In the process flow shown, 17α-hydroxyprogesterone acetate (100mmol, 37.2g), pyridinium p-toluenesulfonate (2mmol, 0.5g) and 300mL tetrahydrofuran were formulated into solution A and placed in a 1L single-port reaction flask; Triethyl formate (200mmol, 29.6g) was dissolved in 120mL ethanol to form solution B, and placed in a 500mL single-necked flask. The materials of solution A and solution B are conveyed by metering pumps respectively, enter the first mixer for mixing, and adjust the flow through the metering pump to make the 17α-hydroxyprogesterone acetate, pyridinium p-toluenesulfonate and orthoformic acid entering the mixer The molar flow ratio of triethyl ester is 1:0.02:2, and the mixed solution in the first mixer enters the first tubular reactor for etherification reaction at 30°C, and stays in the tubular reactor for 30 minutes. The material after the etherification reaction flows out from the first tubular reactor.
[0038] Diphenylamin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com