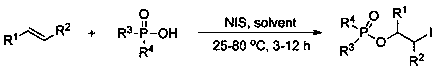Method for preparing 2-iodo-1-phosphoryl substituted alkane compound through efficient olefin bifunctionalization
A compound and phosphoryl technology, applied in the field of application catalytic synthesis of organic phosphate compounds, can solve the problems of harsh reaction conditions, raw material quality and safety, product stability and purity problems, and substrate applicability crossover, etc., to achieve the reaction process. Mild and easy to control, good industrial application prospects, simple and easy to implement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 0.5 mmol of diphenylphosphoric acid, 1.0 mmol of styrene and 0.6 mmol of N - Iodosuccinimide (NIS) was added to the Schlenk tube under nitrogen atmosphere, and 1.0 mL of organic solvent (dichloromethane, dichloroethane, tetrahydrofuran, acetonitrile, toluene, N, N - dimethylformamide, dimethyl sulfoxide, methanol, ethanol, 1,4-dioxane, ethyl acetate), stirred and reacted at room temperature for 12 hours. Through GC detection and analysis, when tetrahydrofuran was used as the reaction solvent, the yield of the difunctionalization reaction was 66%.
Embodiment 2
[0027] 0.5 mmol of diphenylphosphoric acid, 1.0 mmol of styrene and 0.6 mmol of N -Iodosuccinimide was added to the Schlenk tube under nitrogen atmosphere, and 1.0 mL THF was added under nitrogen atmosphere, at the specified temperature (25 o C,40 o C, 60 o C, 80 o C) The reaction was stirred for 12 hours. Analysis by GC detection at 40 o C, the yield of the difunctionalization reaction was 96%.
Embodiment 3
[0029] Mix 0.5 mmol of diphenylphosphoric acid, styrene (0.5 mmol, 0.6 mmol, 0.75 mmol, 1.0 mmol) with 0.6 mmol of N -Iodosuccinimide was added to the Schlenk tube under nitrogen atmosphere, and 1.0 mL THF was added under nitrogen atmosphere, at 40 o The reaction was stirred at C for 12 hours. Through GC detection and analysis, when the amount of styrene was 0.5 mmol, the yield of the difunctionalization reaction was 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com