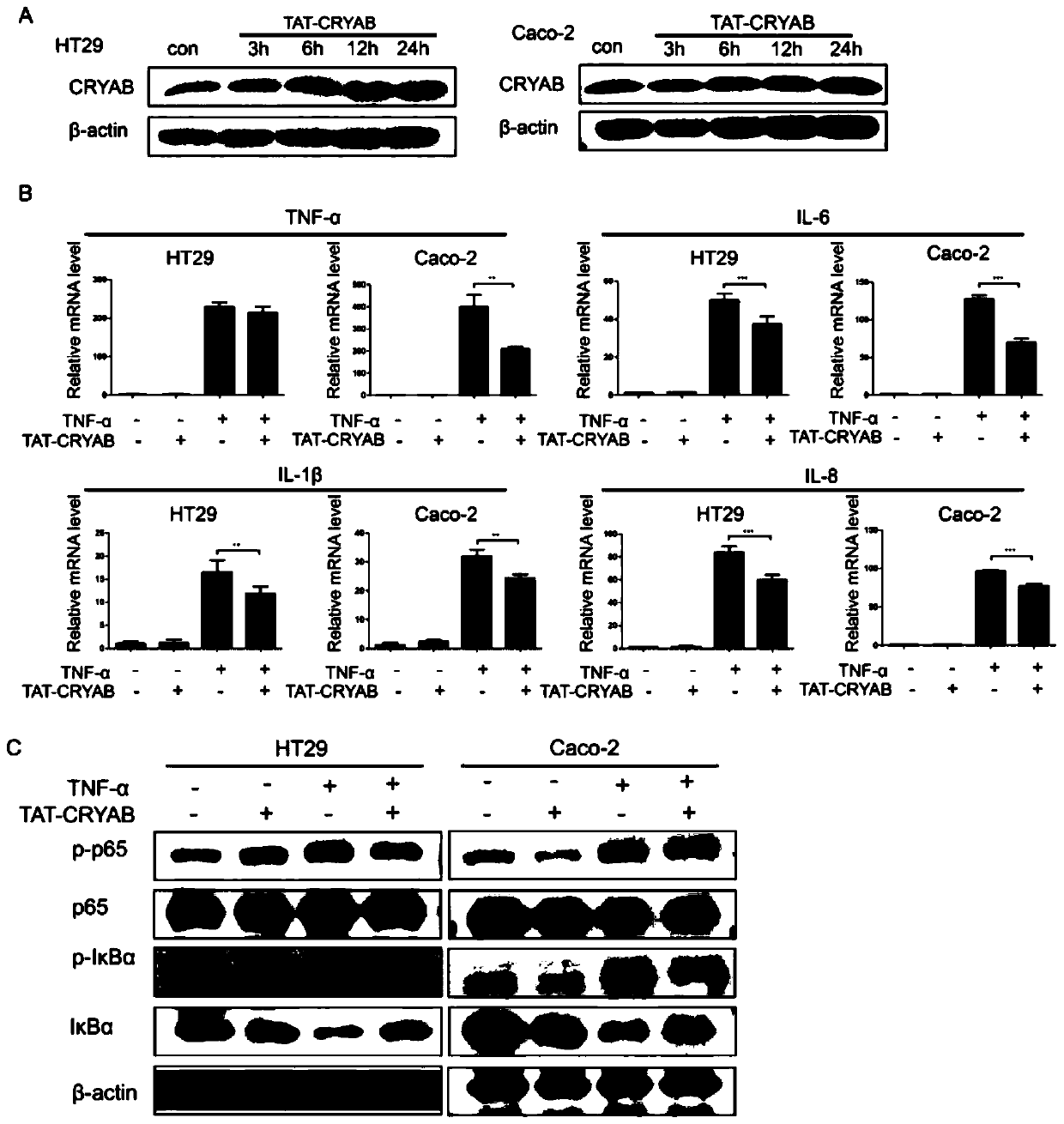
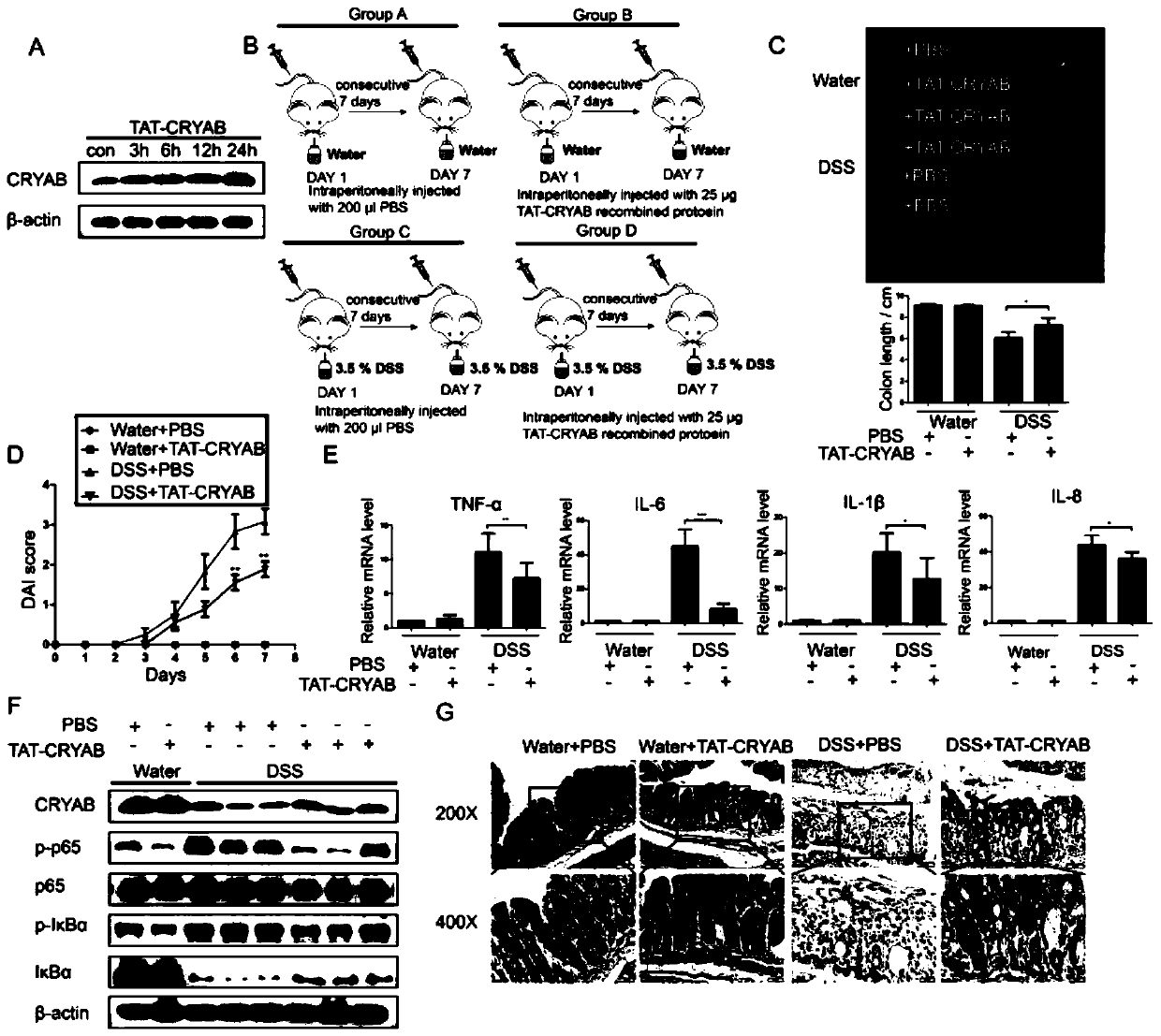
Application method of TAT-CRYAB fusion protein in intestinal inflammation
An intestinal inflammation, fusion protein technology, applied in DNA/RNA fragments, peptide/protein components, chemical instruments and methods, etc., can solve problems such as rare clinical treatment of diseases, and achieve the improvement of disease activity, protection of integrity, inhibition of Effects of Inflammatory Response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] A method for applying TAT-CRYAB fusion protein in intestinal inflammation, specifically comprising the following steps:
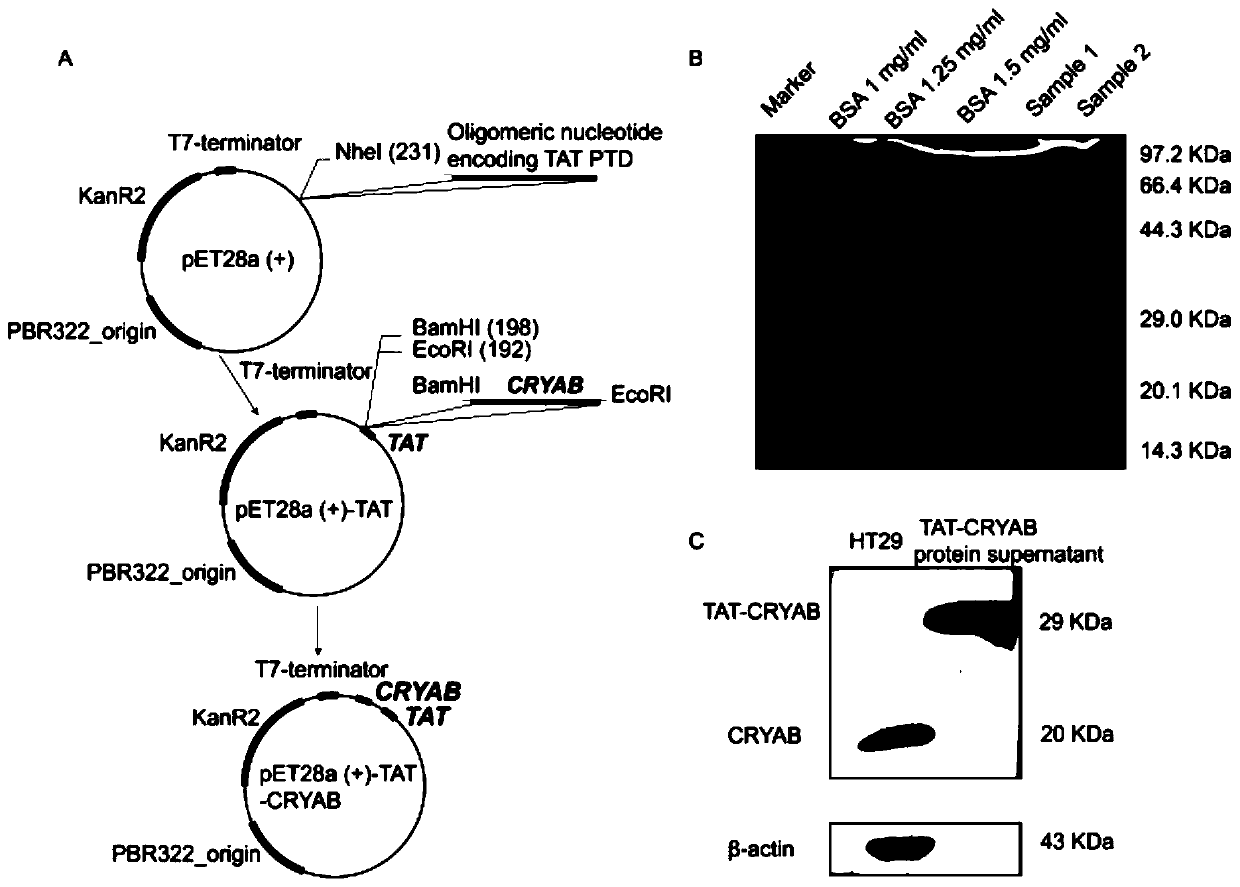
[0050] S1. Construction of pET28a-TAT-CRYAB recombinant plasmid; the construction method is as follows:
[0051] S1.1. Purchase the pET28a plasmid and prepare the pET28a vector digestion system: pET28a vector, NheI, 10xFastdigestBuffer and DEPC water. The details of the pET28a vector digestion system are as follows:
[0052]
[0053] Then prepare the enzyme digestion system, bathe in a 37°C water bath for 1 hour, and then glue it back with the above steps;
[0054] S1.2, pET28a-TAT plasmid connection;
[0055] First, anneal the front and back primers of TAT, and connect after annealing. The system is: 10×T4buffer, T4 ligase, pET28a digestion product and TAT sequence annealing product.
[0056]
[0057] After the system is prepared, let it stand at room temperature for 1 hour;
[0058] S1.3, then transform the pET28a-TAT plasmid, plate, shake...
Embodiment 2
[0079] A method for applying TAT-CRYAB fusion protein in intestinal inflammation, specifically comprising the following steps:
[0080] S1. Construction of pET28a-TAT-CRYAB recombinant plasmid; the construction method is as follows:
[0081] S1.1. Purchase the pET28a plasmid and prepare the pET28a vector digestion system: pET28a vector, NheI, 10xFastdigestBuffer and DEPC water. The details of the pET28a vector digestion system are as follows:
[0082]
[0083] Then prepare the enzyme digestion system, bathe in a 37°C water bath for 1 hour, and then glue it back with the above steps;
[0084] S1.2, pET28a-TAT plasmid connection;
[0085] First, anneal the front and back primers of TAT, and connect after annealing. The system is: 10×T4buffer, T4 ligase, pET28a digestion product and TAT sequence annealing product.
[0086]
[0087] After the system is prepared, let it stand at room temperature for 1 hour;
[0088] S1.3, then transform the pET28a-TAT plasmid, plate, shake...
Embodiment 3
[0108] A method for applying TAT-CRYAB fusion protein in intestinal inflammation, specifically comprising the following steps:
[0109] S1. Construction of pET28a-TAT-CRYAB recombinant plasmid; the construction method is as follows:
[0110] S1.1. Purchase the pET28a plasmid and prepare the pET28a vector digestion system: pET28a vector, NheI, 10xFastdigestBuffer and DEPC water. The details of the pET28a vector digestion system are as follows:
[0111]
[0112] Then prepare the enzyme digestion system, bathe in a 37°C water bath for 1 hour, and then glue it back with the above steps;
[0113] S1.2, pET28a-TAT plasmid connection;
[0114] First, anneal the front and back primers of TAT, and connect after annealing. The system is: 10×T4buffer, T4 ligase, pET28a digestion product and TAT sequence annealing product.
[0115]
[0116] After the system is prepared, let it stand at room temperature for 1 hour;
[0117] S1.3, then transform the pET28a-TAT plasmid, plate, shake...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com