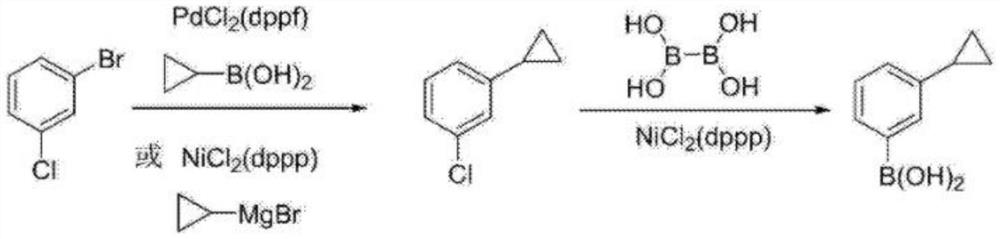
A kind of synthetic method of 3-cyclopropylphenylboronic acid
A technology of cyclopropylphenylboronic acid and cyclopropylboronic acid, which is applied in the field of synthesis of 3-cyclopropylphenylboronic acid, can solve the problems of potential safety hazards, lithiation reagent n-butyllithium is afraid of water and flammability, etc., and meets the equipment requirements Low cost, low cost, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] This embodiment includes the following steps:
[0029] Step 1: 250mL three-neck bottle, equipped with a thermometer and a condenser tube, under nitrogen protection, put 9.57g (0.05mol) of 3-chlorobromobenzene, 70mL of 1,4-dioxane and 15mL of water, start stirring, and pour into Add potassium phosphate 31.84g (0.15mol), PdCl 2 (dppf) 1.83g (0.0025mol) and cyclopropylboronic acid 6.44g (0.075mol). After the feeding is completed, the nitrogen is replaced three times, and the heating is turned on to react at 80°C for 4.0h. Sample delivery, gas phase detection of 3-chlorobromobenzene raw material remains unchanged, stop the reaction, add ethyl acetate for extraction, filter the organic layer with diatomaceous earth, evaporate the solvent to dryness, then heat up and distill under reduced pressure to obtain 3-cyclopropylchlorobenzene 6.94 g (0.045mol), the yield is 91.0%, 1 H NMR (CDCl 3 ,400MHz):δ7.21-7.12(m,2H),7.07(s,1H),6.97(d,J=7.5Hz,1H),1.92-1.86(m,1H),1.03-0.98(m,2...
Embodiment 2
[0032] This embodiment includes the following steps:
[0033] Step 1: 250mL three-neck bottle, equipped with a thermometer and a condenser, under nitrogen protection, put 9.57g (0.05mol) of 3-chlorobromobenzene, 64mL of tetrahydrofuran and 16mL of water, start stirring, and add 20.7g of potassium carbonate to the reaction bottle in turn (0.15mol), PdCl 2 (dppf) 1.10 g (0.0015 mol) and cyclopropylboronic acid 6.44 g (0.075 mol). After the feeding is completed, the nitrogen is replaced three times, and the heating is turned on to react at 60°C for 4.0h. Sample delivery, gas phase detection of 3-chlorobromobenzene raw material remains unchanged, stop the reaction, add ethyl acetate to extract, filter the organic layer with diatomaceous earth, evaporate the solvent to dryness, then heat up and distill under reduced pressure to obtain 3-chlorocyclopropylbenzene 6.71 g (0.043mol), the yield is 88.0%;
[0034] The second step: 250mL three-necked bottle, equipped with a thermometer...
Embodiment 3
[0036] This embodiment includes the following steps:
[0037] Step 1: 250mL three-necked bottle, equipped with a thermometer and a condenser, under nitrogen protection, put 9.57g (0.05mol) of 3-chlorobromobenzene, 65mL of DMF and 15mL of water, start stirring, and add 10.60g of sodium carbonate to the reaction bottle in turn (0.10mol), PdCl 2(dppf) 0.37g (0.0005mol) and cyclopropylboronic acid 4.72g (0.055mol). After the feeding is completed, the nitrogen is replaced three times, and the heating is turned on to react at 120°C for 8.0h. Sample delivery, gas phase detection of 3-chlorobromobenzene raw material remains unchanged, stop the reaction, add ethyl acetate for extraction, filter the organic layer with diatomaceous earth, evaporate the solvent to dryness, then heat up and distill under reduced pressure to obtain 3-chlorocyclopropylbenzene 6.52 g (0.042mol), the yield is 85.5%;
[0038] Step 2: 250mL three-necked bottle, equipped with a thermometer and a condenser, und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com