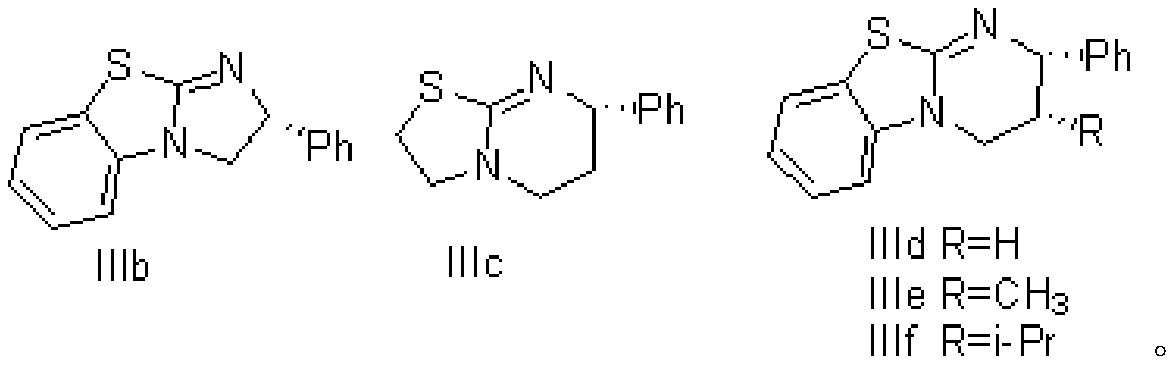Asymmetric synthesis method of L-carnitine
A synthetic method and asymmetric technology, applied in the fields of medicinal chemistry and organic chemistry, can solve the problems of long reaction steps, high risk, toxic tin compounds, etc., and achieve the effect of low cost, simple method and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
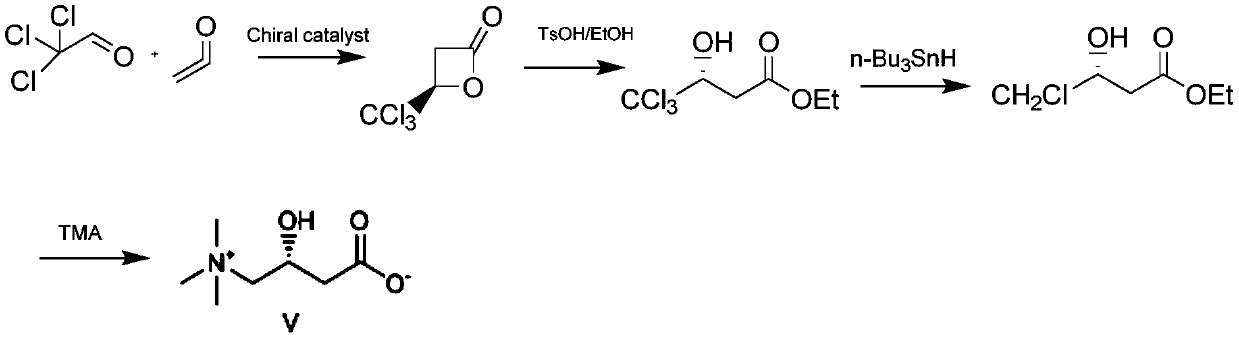
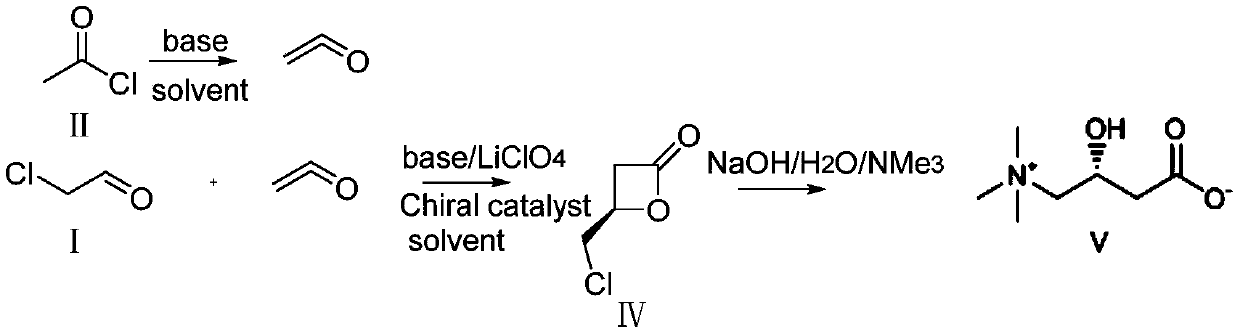
[0026] The synthetic method of L-carnitine is realized through the following steps:
[0027] 1) Preparation of β-chiral lactone
[0028] Under nitrogen protection, add dichloromethane (100ml) and acetyl chloride (39.3g, 0.5mol) to a 500ml double-layer reaction flask (equipped with nitrogen protection, stirring and dropping funnel), and cool the liquid to -50°C. DBU (76.1g, 0.5mol) was added dropwise, the drop was completed, and the temperature was kept for 3 hours, then the 90ml dichloromethane solution dissolved with chloroacetaldehyde (26.2g, 0.33mol) and chiral catalyst IIIb (8.4g, 0.033mol) And lithium perchlorate (10.7g, be dissolved in 89ml dichloromethane and 60mlTHF) solution is added dropwise in the reaction bottle, insulation reaction 1 hour, reaction solution is detected by online infrared, when wave number disappears at 1832 places peak, reaction is completed, reaction Add ethanol (20ml) to the solution to quench, stir for half an hour, then add water, stir for ha...
Embodiment 2
[0033] The synthetic method of L-carnitine is realized through the following steps:
[0034] 1) Preparation of β-chiral lactone
[0035] Under nitrogen protection, add dichloromethane (100ml) and acetyl chloride (39.3g, 0.5mol) to a 500ml double-layer reaction flask (equipped with nitrogen protection, stirring and dropping funnel), and cool the liquid to -50°C. DBU (76.1g, 0.5mol) was added dropwise, and the dropwise temperature was maintained for 3 hours, then the 90ml dichloromethane solution dissolved with chloroacetaldehyde (26.2g, 0.33mol) and chiral catalyst IIIc (7.3g, 0.033mol) And lithium perchlorate (10.7g, be dissolved in 89ml dichloromethane and 60mlTHF) solution is added dropwise in the reaction bottle, insulation reaction 1 hour, reaction solution is detected by online infrared, when wave number disappears at 1832 places peak, reaction is completed, reaction Add ethanol (20ml) to the solution, stir for half an hour, then add water, stir for half an hour, take it...
Embodiment 3
[0056] The synthetic method of L-carnitine is realized through the following steps:
[0057] 1) Preparation of β-chiral lactone
[0058] Under nitrogen protection, add dichloromethane (100ml) and acetyl chloride (39.3g, 0.5mol) to a 500ml double-layer reaction flask (equipped with nitrogen protection, stirring and dropping funnel), and cool the liquid to -50°C. DBU (76.1g, 0.5mol) was added dropwise, the drop was completed, and the temperature was kept for 3 hours, then 90ml of dichloromethane solution dissolved with chloroacetaldehyde (26.2g, 0.33mol) and chiral catalyst IIId (8.9g, 0.033mol) And the solution of lithium perchlorate (10.7g, being dissolved in 89ml dichloromethane and 60mlTHF) is added dropwise in reaction bottle, insulation reaction 1 hour, reaction solution is detected by online infrared, when wave number disappears at 1832 place peaks, reaction completes, Add ethanol (20ml) to the reaction solution, stir for half an hour, then add water, stir for half an ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com