A kind of copper catalyzed method for synthesizing β-keto ester
A ketoester, copper-catalyzed technology, applied in the field of synthetic chemistry, can solve the problems of unstable diketene, harsh reaction conditions, complex products, etc., and achieve the effects of good substrate universality, mild reaction conditions, and wide substrate range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
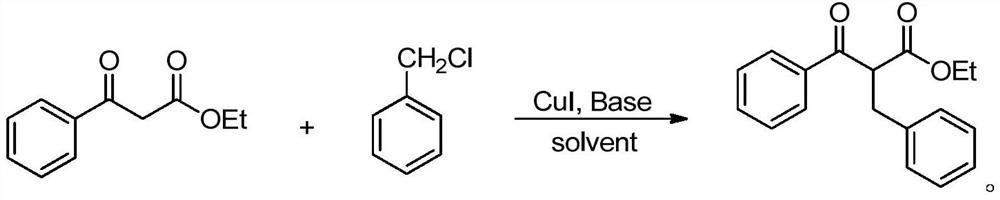
[0023]
[0024] Into the reaction tube were sequentially added ethyl benzoyl acetate (1.0 mmol), benzyl chloride (1.0 mmol), catalyst CuI (0.05 mmol), K 2 CO 3 (0.06 mmol), then 2 mL of solvent toluene was added, and the reaction was carried out at 60° C. for 12 hours. After the reaction, the reaction solution was concentrated, and the corresponding product was obtained by column chromatography, and the isolated yield was 87%. 1 H NMR (400MHz, CDCl 3 )δ: 1.21(t, J=7.5Hz, 3H), 2.73(dd, J=5.0, 17.0Hz, 1H), 3.38(q, J=10.0Hz, 1H), 4.11(q, J=7.5Hz, 2H), 5.11(q, J=5.0Hz, 1H), 7.21~7.25(m, 1H), 7.17(d, J=4.5Hz, 4H), 7.38~7.41(m, 2H), 7.49(tt, J =1.5,7.5Hz,1H),7.98~8.00(m,2H).
Embodiment 2
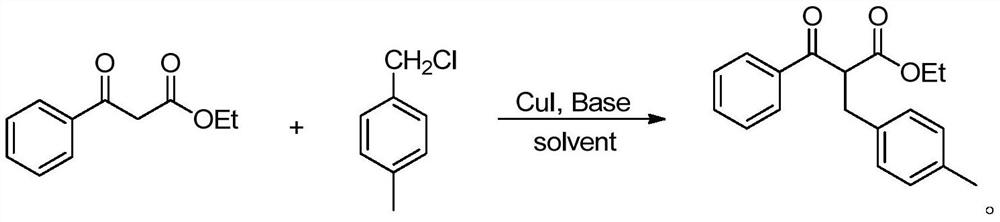
[0026]
[0027] Into the reaction tube were sequentially added ethyl benzoyl acetate (1.0 mmol), 4-methylbenzyl chloride (1.2 mmol), catalyst CuI (0.05 mmol), Cs 2 CO 3 (0.08 mmol), then 2 mL of solvent toluene was added, and the reaction was carried out at 80° C. for 16 hours. After the reaction, the reaction solution was concentrated, and the corresponding product was obtained by column chromatography, and the isolated yield was 89%. 1 H NMR (400MHz, CDCl 3 )δ: 1.21(t, J=7.0Hz, 3H), 2.29(s, 3H), 2.71(dd, J=5.0, 17.0Hz, 1H), 3.36(dd, J=10.0, 17.0Hz, 1H), 4.11(q,J=7.0Hz,2H),5.07(q,J=5.0,10.0Hz,1H),7.10(d,J=8.0Hz,2H),7.19(d,J=8.0Hz,2H), 7.39 (t, J=7.5Hz, 2H), 7.39 (t, J=7.5Hz, 1H), 7.98 (d, J=7.0Hz, 2H).
Embodiment 3
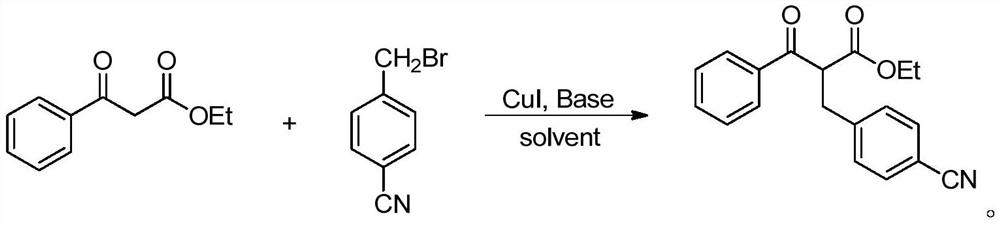
[0029]
[0030] Into the reaction tube were sequentially added ethyl benzoyl acetate (1.0 mmol), 4-cyanobenzyl bromide (1.2 mmol), catalyst cuprous iodide CuI (0.05 mmol), Cs 2 CO 3 (0.10 mmol), then 2 mL of solvent toluene was added, and the reaction was carried out at 90° C. for 18 hours. After the reaction was completed, the reaction solution was concentrated, and the corresponding product was obtained by column chromatography. The isolated yield was 93%. 1 H NMR (400MHz, CDCl 3 )δ: 1.21 (t, J=7.1Hz, 3H), 2.74 (dd, J=5.5, 17.0Hz, 1H), 3.70 (dd, J=9.3, 17.0Hz, 1H), 4.11 (q, J=7.2 Hz,2H),5.18(dd,J=5.5,9.3Hz,1H),7.42~7.46(m,4H),7.54(t,J=7.4Hz,1H),7.60~7.61(m,2H),7.94 ~7.96 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com