Diltiazem hydrochloride tablet and preparation method thereof
A technology of diltiazem hydrochloride tablets and diltiazem, which can be applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the problems of easy residual organic solvents, low production efficiency and dissolution rate. It can reduce the frequency of taking medication, improve the output rate, and simplify the production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
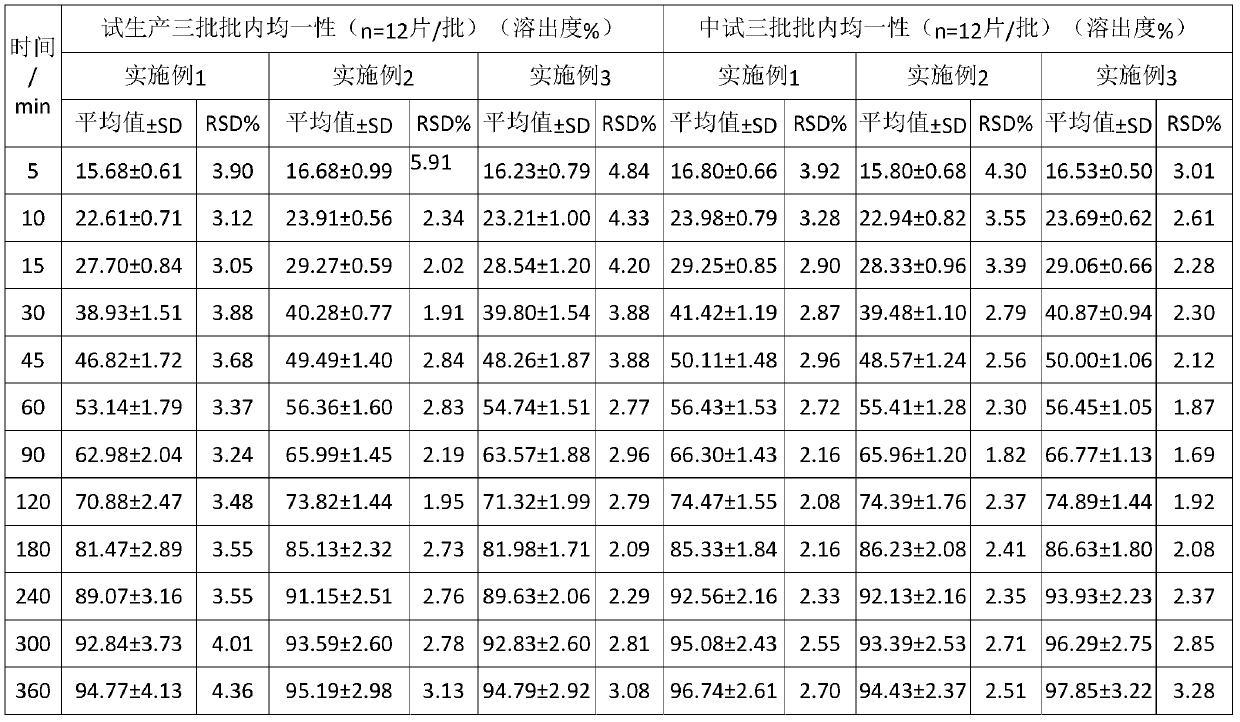
Embodiment 1
[0035] This embodiment provides a kind of diltiazem hydrochloride tablets, the diltiazem hydrochloride Tablets are prepared from the following raw materials comprising by weight:
[0036] diltiazem hydrochloride 15%; filler 50%; polyethylene glycol 6000 10%; hydrogenated castor oil 10%; microcrystalline cellulose 10%, and the filler is calcium hydrogen phosphate.
[0037] diltiazem hydrochloride The preparation method of sheet, comprises the following steps:
[0038] (1) diltiazem hydrochloride , filler, polyethylene glycol, hydrogenated castor oil and microcrystalline cellulose are mixed and then melted, granulated and sieved to form a granulated semi-finished product, cooled for later use;
[0039] (2) compress the semi-finished product obtained in step (1), or mix the semi-finished product with a lubricant and then compress the tablet to obtain the diltiazem hydrochloride piece.
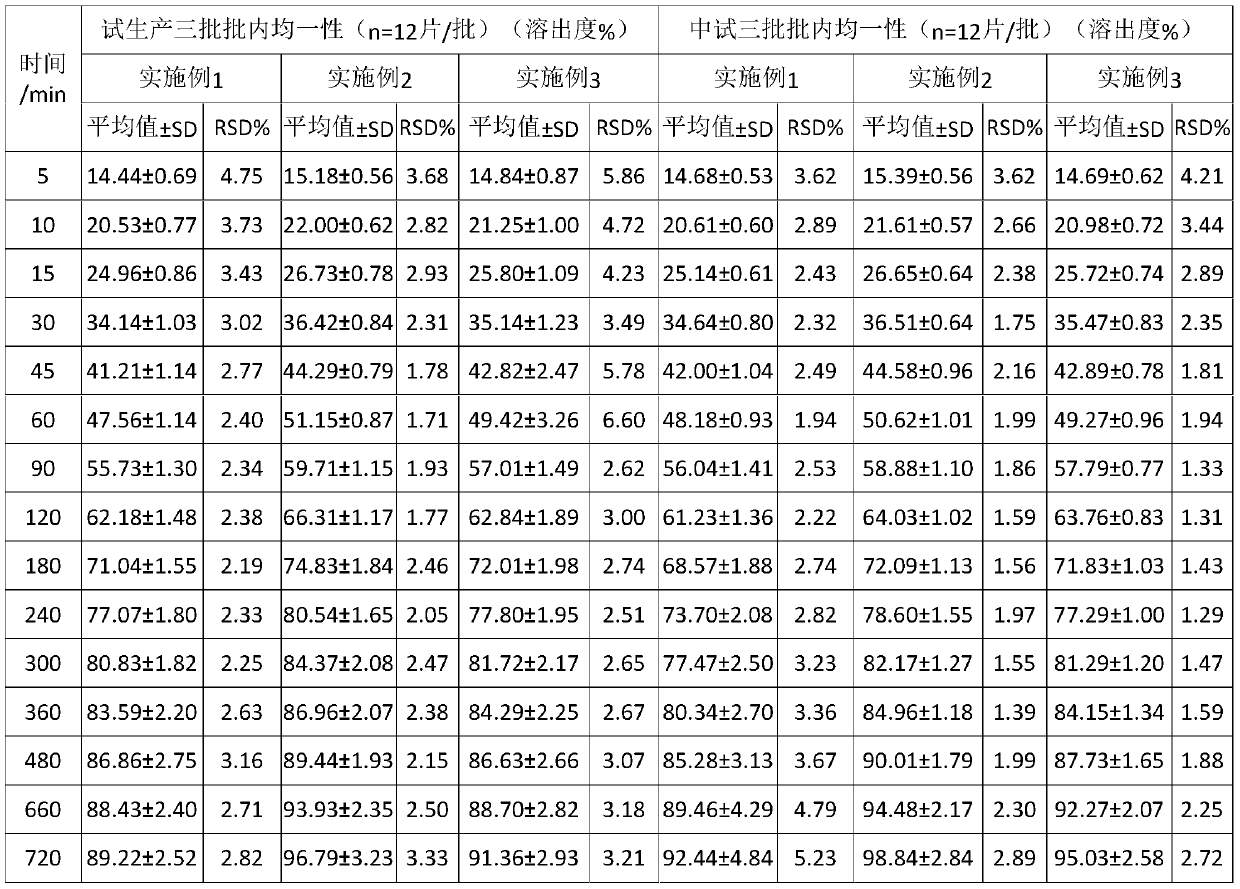
Embodiment 2
[0041] This embodiment provides a kind of diltiazem hydrochloride tablets, the diltiazem hydrochloride Tablets are prepared from the following raw materials comprising by weight:
[0042] diltiazem hydrochloride 15%; filler 44.6%; polyethylene glycol 4000 5%; hydrogenated castor oil 30%; microcrystalline cellulose 5%, the filler is lactose, lubricant 0.4%, and the lubricant is talcum powder.
[0043] diltiazem hydrochloride The preparation method of sheet, comprises the following steps:
[0044] (1) diltiazem hydrochloride , filler, polyethylene glycol, hydrogenated castor oil and microcrystalline cellulose are mixed and put into a mixing granulator to melt and granulate, and pass through a 18-mesh sieve to form a granular semi-finished product, which is cooled by natural cooling for standby;
[0045] (2) Tablet the semi-finished product obtained in step (1), or use a high-speed mixer to mix the semi-finished product and lubricant, and then compress the tablet to obt...
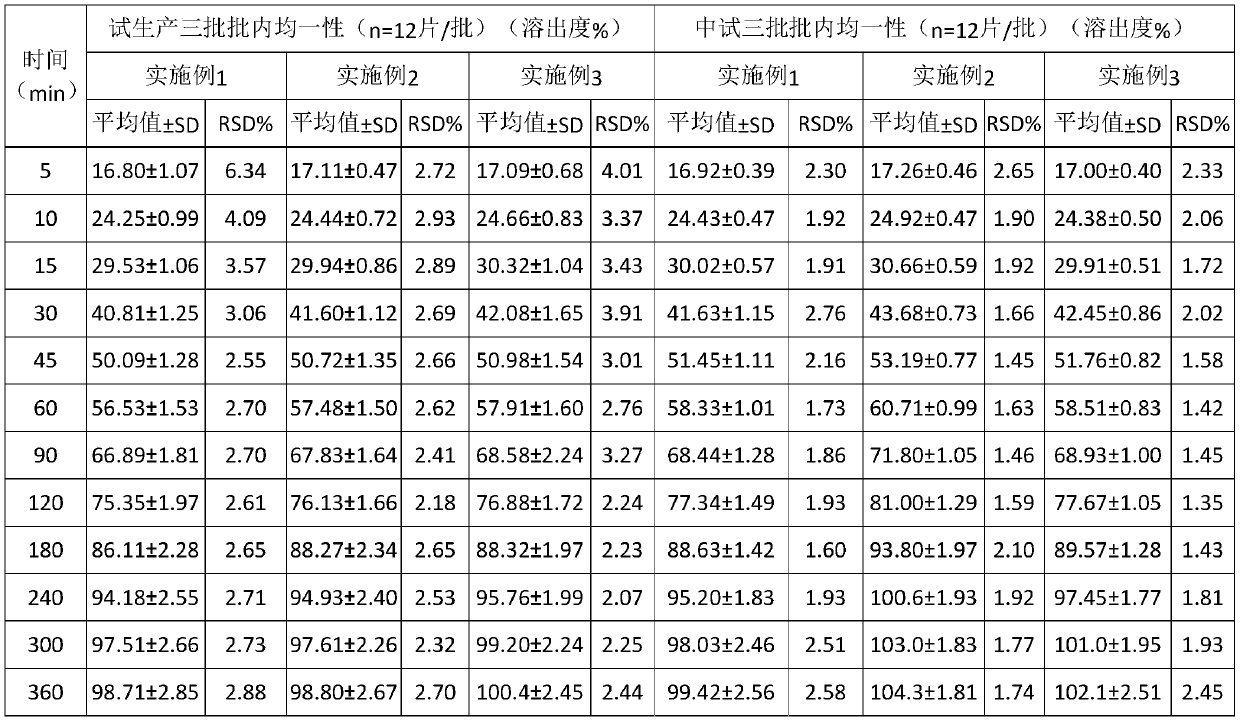
Embodiment 3
[0047] This embodiment provides a kind of diltiazem hydrochloride tablets, the diltiazem hydrochloride Tablets are prepared from the following raw materials comprising by weight:
[0048] diltiazem hydrochloride 15%; filler 59%; polyethylene glycol 6000 is 10%; hydrogenated castor oil 10%; microcrystalline cellulose 5%, the filler is calcium carbonate, lubricant 1%, and the lubricant is talcum powder , magnesium stearate and stearic acid, the weight ratio of described talcum powder, magnesium stearate and stearic acid 4:2:3.
[0049] diltiazem hydrochloride The preparation method of sheet, comprises the following steps:
[0050] (1) diltiazem hydrochloride , filler, polyethylene glycol, hydrogenated castor oil and microcrystalline cellulose are mixed and put into a mixing granulator to melt and granulate, pass through a 18-mesh sieve to form a granulated semi-finished product, and use natural air in a hot air circulation oven or cold wind to cool down, spare;
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com