Synthesis method of Beraprost sodium intermediate
A synthesis method and compound technology, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as hidden dangers of industrial production safety, equipment is difficult to meet requirements, and the reaction environment is unfriendly, and the reaction time can be shortened, It is convenient for safe operation and industrial production, and the effect of saving operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
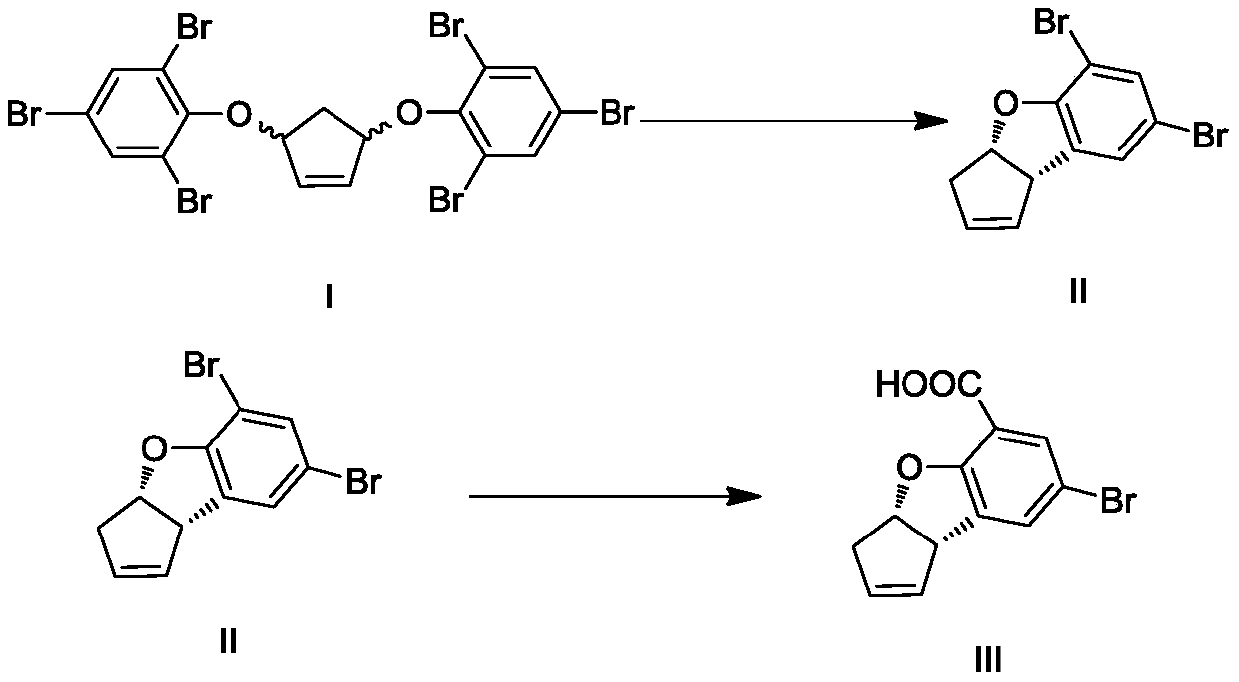
[0056] Embodiment one: the synthesis of compound I
[0057]
[0058] Add cyclopentene, carbon tetrachloride, NBS, benzoyl peroxide (BPO), cyclopentene and carbon tetrachloride in a mass / volume ratio of 1:10 g / mL, cyclopentene and NBS molar The ratio is 1:1.8, the molar ratio of cyclopentene and benzoyl peroxide (BPO) is 1:0.01, the system is under nitrogen atmosphere, at 60-70°C, react for 0.5-1 hour, cool down to 0-5°C and stir At least half an hour. Then filter, collect the filtrate, and directly carry out the next reaction.
[0059] In another reaction flask, add ethylene glycol dimethyl ether, add 60% NaH, the molar ratio of cyclopentene and NaH is 1:1.8, cool down to -10~0°C, add 2,4,6-tribromophenol Ethylene glycol dimethyl ether solution, the molar ratio of cyclopentene and tribromophenol is 1:1.8, stir at -5~0°C for at least 1 hour, and add the previously prepared dibromide solution at -5~0°C , add 18-crown-6, rise to room temperature, and stir the reaction at ro...
Embodiment 2
[0064] Embodiment two: the synthesis of compound I
[0065] Add cyclopentene, carbon tetrachloride, NBS, benzoyl peroxide (BPO), cyclopentene and carbon tetrachloride in a mass / volume ratio of 1:10 g / mL, cyclopentene and NBS molar The ratio is 1:1.8, the molar ratio of cyclopentene and benzoyl peroxide (BPO) is 1:0.03, the system is under nitrogen atmosphere, at 70-76°C, reacted for 0.5-1 hour, cooled to 0-5°C, Stir for at least half an hour. Then filter, collect the filtrate, and directly carry out the next reaction.
[0066] In another reaction flask, add ethylene glycol dimethyl ether, add 60% NaH, the molar ratio of cyclopentene and NaH is 1:1.8, cool down to -10~0°C, add 2,4,6-tribromophenol Ethylene glycol dimethyl ether solution, the molar ratio of cyclopentene and tribromophenol is 1:1.8, stir at -5~0°C for at least 1 hour, and add the previously prepared dibromide solution at -5~0°C , add 18-crown-6, rise to room temperature, and stir the reaction at room temperatu...
Embodiment 3
[0067] Embodiment three: the synthesis of compound I
[0068] Add cyclopentene, carbon tetrachloride, NBS, benzoyl peroxide (BPO), cyclopentene and carbon tetrachloride in a mass / volume ratio of 1:20 g / mL, cyclopentene and NBS molar The ratio is 1:1.8, the molar ratio of cyclopentene and benzoyl peroxide (BPO) is 1:0.01, the system is under nitrogen atmosphere, at 76-80°C, react for 0.5-1 hour, cool down to 0-5°C and stir At least half an hour. Then filter, collect the filtrate, and directly carry out the next reaction.
[0069]In another reaction flask, add ethylene glycol dimethyl ether, add 60% NaH, the molar ratio of cyclopentene and NaH is 1:2.0, cool down to -10~0°C, add 2,4,6-tribromophenol Ethylene glycol dimethyl ether solution, the molar ratio of cyclopentene and tribromophenol is 1:2.0, stir at -5~0°C for at least 1 hour, and add the previously prepared dibromide solution at -5~0°C , add 18-crown-6, rise to room temperature, and stir the reaction at room temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com