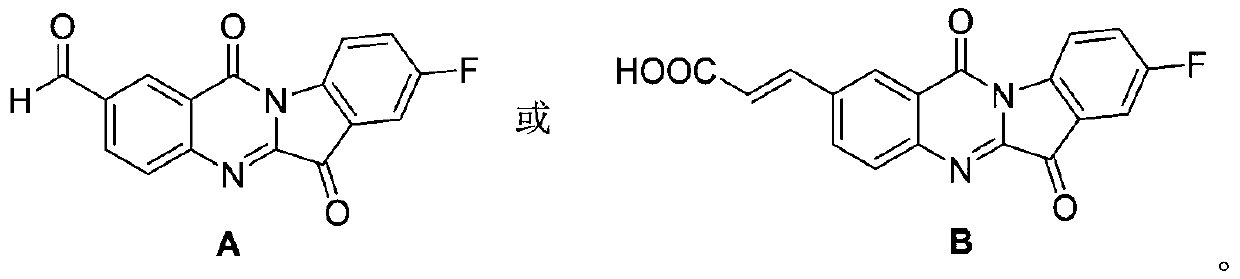
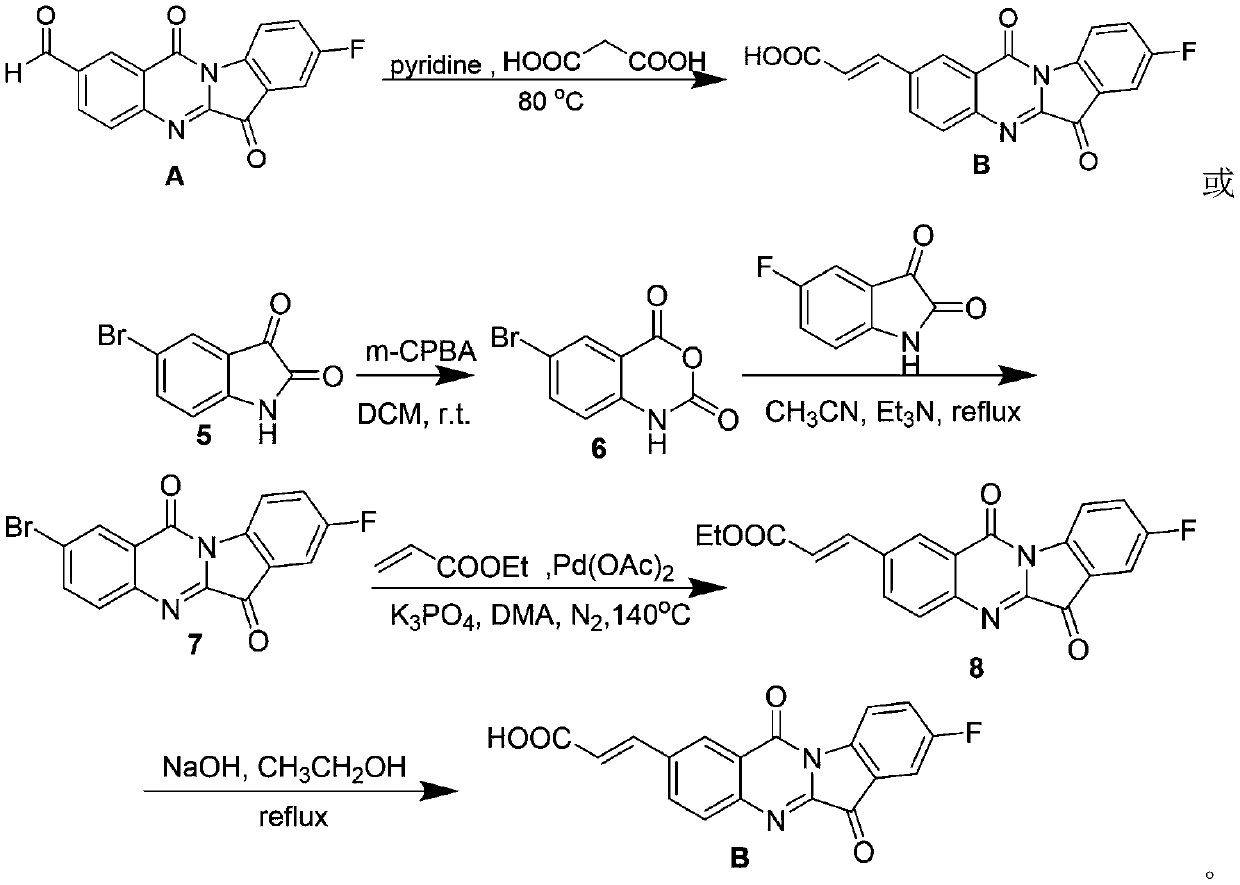
Tryptanthrin derivative containing olefine acid, and preparation method and application thereof
A derivative, the technology of tryptanthrin, which is applied in the field of preparation of tryptanthrin derivatives, achieves the effects of simple operation, increased water solubility, and easy industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
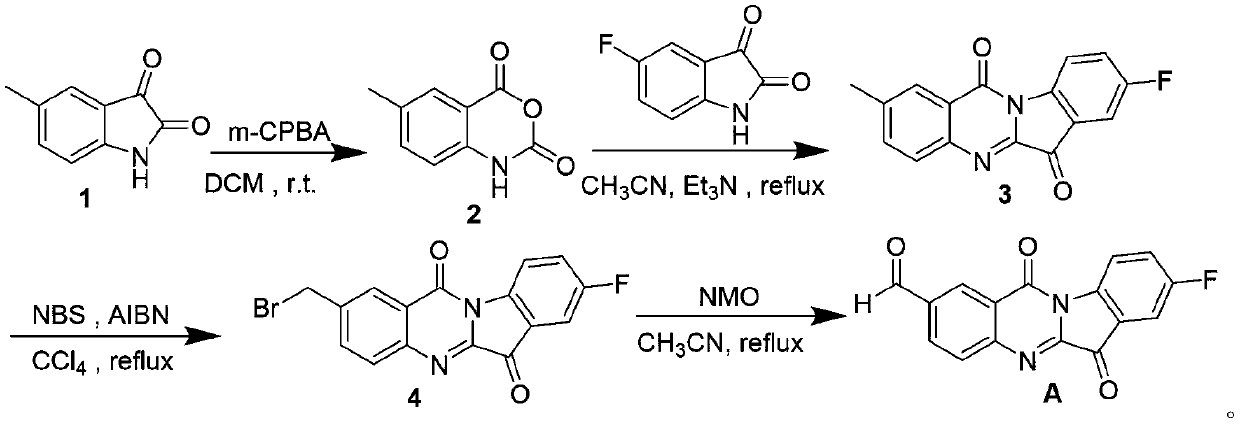
[0064] Step 1: Synthesis of 5-methylisatoic anhydride
[0065]
[0066] Suspend 1000.0 mg (6.213 mmol) of 5-methylisatin 1 in 20 mL of dry dichloromethane, add 2293.5 mg (12.43 mmol) of m-chloroperoxybenzoic acid at 0°C, and stir at room temperature for 2 to 4 Hour; After the TLC detection reaction was completed, the orange solid obtained by filtering the reaction solution was washed with (5mL×3) ethyl acetate to obtain 5-methylisatoic anhydride 2;
[0067] Step 2: Synthesis of 2-methyl-8-fluorotryptanthrin
[0068]
[0069] Suspend 1000.0mg (5.650mmol) of 5-methylisatoic anhydride 2 and 932.9mg (5.650mmol) of 5-fluoroisatin in 10mL of dry acetonitrile solvent, add 2.35mL of triethylamine, heat and stir for 82~ Reflux at 90°C for 4 to 4.5 hours, cool to room temperature, filter, and wash the filter cake with (5mL×3) methanol to obtain a yellow-green solid, namely 2-methyl-8-fluorotryptanthin 3;
[0070] Characterization data:
[0071] 1 HNMR (600MHz, CDCl 3 )δ8.63(d...
Embodiment 2
[0083] Step 1: Synthesis of 5-methylisatoic anhydride
[0084]
[0085] Suspend 1000.0 mg (6.213 mmol) of 5-methylisatin 1 in 20 mL of dry dichloromethane, add 2293.5 mg (12.43 mmol) of m-chloroperoxybenzoic acid at 0°C, and stir at room temperature for 2 to 4 Hour; After the TLC detection reaction was completed, the orange solid obtained by filtering the reaction solution was washed with (5mL×3) ethyl acetate to obtain 5-methylisatoic anhydride 2;
[0086] Step 2: Synthesis of 2-methyl-8-fluorotryptanthrin
[0087]
[0088] Suspend 1000.0mg (5.650mmol) of 5-methylisatoic anhydride 2 and 932.9mg (5.650mmol) of 5-fluoroisatin in 10mL of dry acetonitrile solvent, add 2.35mL of triethylamine, heat and stir for 82~ Reflux at 90°C for 4 to 4.5 hours, cool to room temperature, filter, and wash the filter cake with (5mL×3) methanol to obtain a yellow-green solid, namely 2-methyl-8-fluorotryptanthin 3;
[0089] Step 3: Synthesis of 2-bromomethyl-8-fluorotryptanthrin
[0090] ...
Embodiment example 3
[0096] Step 1: Synthesis of 5-methylisatoic anhydride
[0097]
[0098] Suspend 1000.0 mg (6.213 mmol) of 5-methylisatin 1 in 20 mL of dry dichloromethane, add 2293.5 mg (12.43 mmol) of m-chloroperoxybenzoic acid at 0°C, and stir at room temperature for 2 to 4 Hour; After the TLC detection reaction was completed, the orange solid obtained by filtering the reaction solution was washed with (5mL×3) ethyl acetate to obtain 5-methylisatoic anhydride 2;
[0099] Step 2: Synthesis of 2-methyl-8-fluorotryptanthrin
[0100]
[0101] Suspend 1000.0mg (5.650mmol) of 5-methylisatoic anhydride 2 and 932.9mg (5.650mmol) of 5-fluoroisatin in 10mL of dry acetonitrile solvent, add 2.35mL of triethylamine, heat and stir for 82~ Reflux at 90°C for 4 to 4.5 hours, cool to room temperature, filter, and wash the filter cake with (5mL×3) methanol to obtain a yellow-green solid, namely 2-methyl-8-fluorotryptanthin 3;
[0102] Step 3: Synthesis of 2-bromomethyl-8-fluorotryptanthrin
[0103] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com