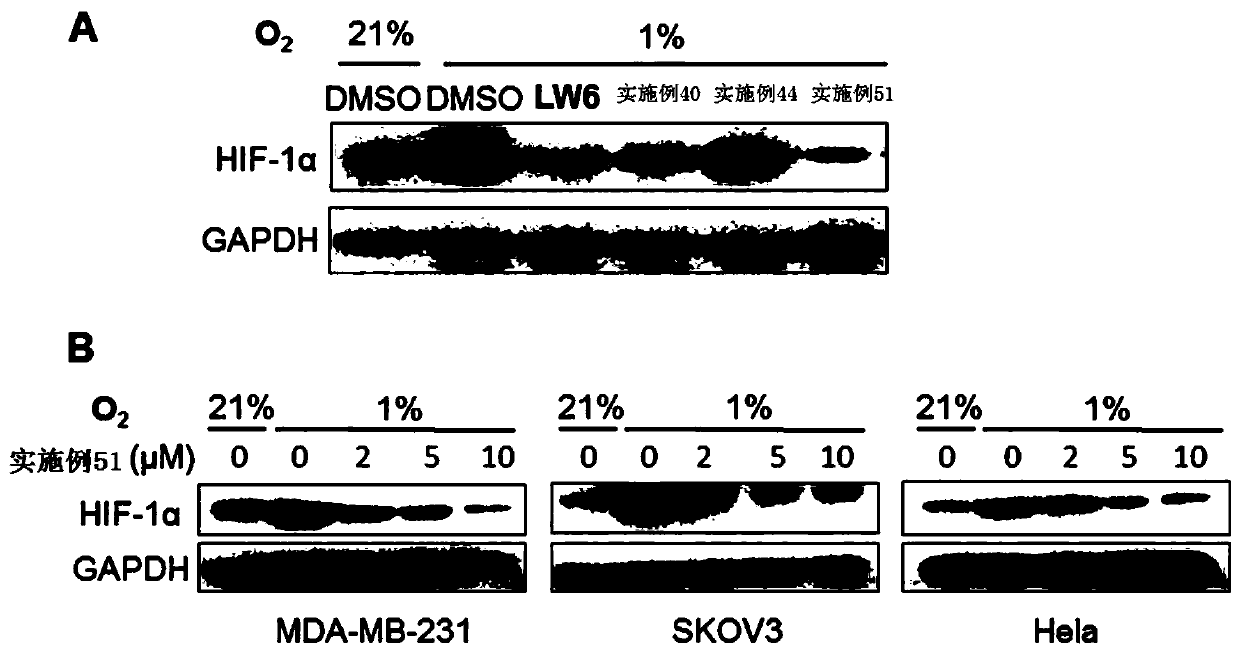
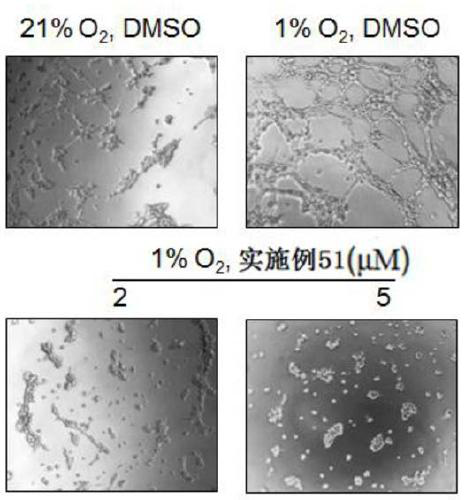
Aryl formamide compound and preparation method, pharmaceutical composition and application thereof
A technology for aryl formamides and compounds, applied in the field of aryl formamide compounds and their preparation, to achieve the effects of inhibiting aggregation, inhibiting the generation of VEGF, and inhibiting migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Synthesis of N-(2-chlorophenethyl)-2-methyl-thiazole-4-carboxamide
[0042] The reaction formula is as follows:
[0043] The synthesis steps are as follows:
[0044] (1) Dissolve 2-methylthiazole-4-carboxylic acid (1mmol) in 5mL of dichloromethane solution at 0°C, slowly add oxalyl chloride (0.17mL) and N,N-dimethylformaldehyde Amide (1 drop), add and react at room temperature for 1 hour, and after the reaction is completed, the solvent is distilled off under reduced pressure to obtain a residue;
[0045] (2) Dissolve the residue in 2 mL of dichloromethane, add 2-(2-chlorophenyl)ethylamine (1 mmol), dichloromethane (5 mL) and triethylamine (0.16 mL) into a mixed solution, and stir at room temperature 24h. After the completion of the reaction was confirmed by TLC analysis, the solvent was distilled off under reduced pressure, and the residue was purified by flash column chromatography to obtain the target compound as a colorless oil with a yield of 84.3%.
...
Embodiment 2
[0047] Example 2 Synthesis of N-(2-chlorophenethyl)-5-methyl-furan-2-carboxamide
[0048] Choose 5-methylfuran-2-carboxylic acid as the raw material, and other embodiments are the same as in Example 1.
[0049] The prepared compound was a colorless oil with a yield of 67.0%.
[0050] 1 HNMR (600MHz, CDCl 3 )δ7.37(dd, J=7.5, 1.5Hz, 1H), 7.28-7.26(m, 1H), 7.22-7.17(m, 2H), 6.99(d, J=3.3Hz, 1H), 6.37(brs , 1H), 6.08(d, J=3.3Hz, 1H), 3.68(td, J=7.0Hz, 2H), 3.05(t, J=7.0Hz, 2H), 2.32(s, 3H); 13 CNMR (151MHz, CDCl 3 )δ158.59, 154.32, 146.38, 136.51, 134.13, 131.00, 129.59, 128.02, 126.96, 115.28, 108.43, 38.77, 33.62, 13.74; ESI-HRMS (m / z) calcdC 14 h 14 ClNO 2 (M+H + ) 264.0786, found 264.0789.
Embodiment 3
[0051] Example 3 Synthesis of N-(2-chlorophenethyl)-2-(pyridin-3-yl)-thiazole-4-carboxamide
[0052] Select 2-(pyridin-3-yl)-thiazole-4-carboxylic acid as the raw material, and other embodiments are the same as in Example 1.
[0053] The prepared compound was a white solid with a yield of 74.1%.
[0054] 1 HNMR (600MHz, CDCl 3 )δ9.16(s, 1H), 8.70(d, J=5.4Hz, 1H), 8.19(d, J=7.8Hz, 1H), 8.15(s, 1H), 7.42-7.38(m, 2H), 7.30(dJ=7.8Hz, 1H), 7.24-7.19(m, 2H), 3.77(t, J=7.0Hz, 2H), 3.12(t, J=7.0Hz, 2H); 13 C NMR (151MHz, CDCl 3 )δ164.08, 160.22, 150.72, 150.65, 147.08, 135.84, 133.59, 133.11, 130.43, 129.08, 128.26, 127.55, 126.39, 123.16, 122.88, 38.54, 32.90; 17 h 14 ClN 3 OS(M+H + )344.0619, found344.0618.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com