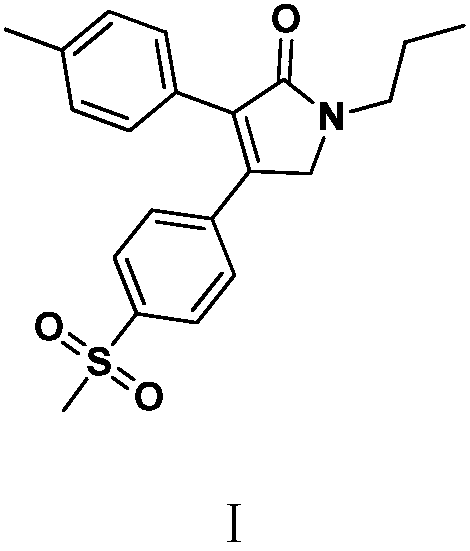
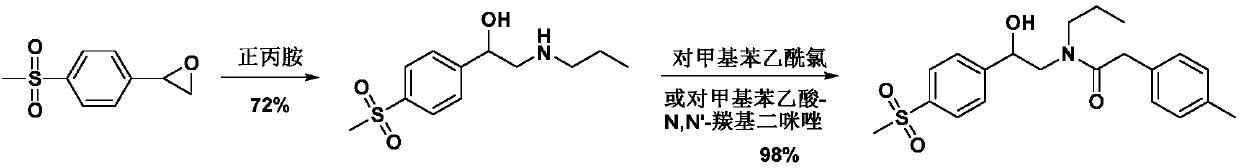
Preparation method of imrecoxib
A compound and solvent technology, applied in the field of medicinal chemistry, can solve the problems of cumbersome preparation process and purification method, which is not conducive to ensuring product quality, and cannot further reduce the price, and achieves the effect of low cost, low equipment requirements and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Preparation of N-n-propyl-N-p-methylsulfonylbenzoylmethyl-4-methylphenylacetamide (Ⅳ)
[0048] In a 1000 ml four-neck flask connected with a stirring, thermometer, and distillation system, add 350 g of toluene, 127.5 g (0.5 mole) mol) methyl 4-methylphenylacetate (Ⅲ1), stirred and reacted at 85 to 90° C. for 5 hours, while distilling out the by-product methanol. The liquid phase detection reaction was complete, the solvent was recovered by distillation under reduced pressure, 400 g of n-hexane was added to the residue, recrystallized, filtered, and dried to obtain 190.6 g of white solid N-n-propyl-N-p-methylsulfonylbenzoylmethyl Base-4-methylphenylacetamide (Ⅳ), the yield is 98.5%, and the liquid phase purity is 99.7%.
Embodiment 2
[0049] Example 2: Preparation of N-n-propyl-N-p-methylsulfonylbenzoylmethyl-4-methylphenylacetamide (Ⅳ)
[0050] Add 300 grams of N,N-dimethylformamide and 127.5 grams (0.5 moles) of ɑ-n-propylamino-4-methylsulfonyl acetophenone to a 1000-milliliter four-necked flask connected with a stirring, thermometer, and distillation system (II), 90.8 g (0.51 moles) of ethyl 4-methylphenylacetate (III2), stirred and reacted at 95 to 100° C. for 4 hours, while distilling out by-product ethanol. The liquid phase detection reaction was complete, the solvent was recovered by distillation under reduced pressure, 400 g of n-hexane was added to the residue, recrystallized, filtered, and dried to obtain 190.2 g of white solid N-n-propyl-N-p-methylsulfonylbenzoylmethyl Base-4-methylphenylacetamide (Ⅳ), the yield is 98.3%, and the liquid phase purity is 99.8%.
Embodiment 3
[0051] Example 3: Preparation of N-n-propyl-N-p-methylsulfonylbenzoylmethyl-4-methylphenylacetamide (Ⅳ)
[0052] Add 60 grams of N,N-dimethylformamide and 25.5 grams (0.1 moles) of α-n-propylamino-4-methylsulfonyl acetophenone to a 500-milliliter four-necked flask connected with a stirring, thermometer, and distillation system (II), 21.6 g (0.1 mol) of tert-butyl 4-methylphenylacetate (III3), stirred and reacted at 95 to 100° C. for 4 hours, while distilling off by-product tert-butyl alcohol. The liquid phase detection reaction was complete, the solvent was recovered by distillation under reduced pressure, 80 g of n-hexane was added to the residue, recrystallized, filtered, and dried to obtain 37.5 g of white solid N-n-propyl-N-p-methylsulfonylbenzoylmethyl Base-4-methylphenylacetamide (Ⅳ), the yield is 96.9%, and the liquid phase purity is 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com