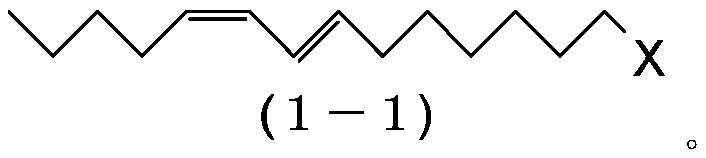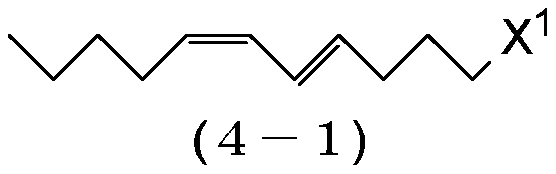1-haloalkadiene and process for preparing same and process for preparing (9e,11z)-9,11-hexadecadienyl acetate
A technology of carbadienyl acetate and halogenated diene, applied in the field of 1-halogenated diene and its preparation and (9E,11Z)-9,11-hexadecadienyl acetate, Can solve problems such as outbreaks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0223] Preparation of (7E,9Z)-1-chloro-7,9-tetradecadiene (1-1:X=C1)
[0224] 1-Bromopentane (185g, 1.20mol), triphenylphosphine (321g, 1.20mol) and N,N-dimethylformamide (225g) were added to the reactor, and the resulting mixture was heated at 110-115°C Stir for 6 hours to prepare pentyltriphenylphosphonium bromide. The reaction mixture was cooled to 20˜30° C., and tetrahydrofuran (967 g) was added thereto. Then, it was cooled to 0˜10° C., and potassium tert-butoxide (139 g, 1.20 mol) was added thereto. Stir at 10-15°C for 30 minutes, then add (2E)-9-chloro-2-nonenal (2-1: X=C1) (171 g, 0.978 mol) dropwise at -5 to 5°C. After the dropwise addition was complete, the reaction mixture was stirred for 3 hours and then quenched by adding water (593 g) to the reaction mixture. After removing the aqueous layer by liquid-liquid separation, the organic layer was concentrated by evaporating the solvent in vacuo. Then, hexane (713 g) was added to precipitate triphenylphosphine oxi...
Embodiment 2
[0230] Preparation of (8E,10Z)-1-chloro-8,10-pentadecadiene (1-3:X=C1)
[0231] Magnesium (17.8 g, 0.733 g atom) and tetrahydrofuran (198 g) were added to the reactor, and the resulting mixture was stirred at 60-65° C. for 30 minutes. Then, (4E,6Z)-1-chloro-4,6-undecadadiene (4-1:X1=Cl) (124g, 0.667mol) was added dropwise at 60-70°C, and the resulting mixture was heated at 70 Stir at ~75°C for 6 hours to prepare (4E,6Z)-4,6-undecadienylmagnesium chloride. In another reactor was charged cuprous iodide (1.27 g, 0.00667 mol), triethyl phosphite (2.66 g, 0.0160 mol), 1-bromo-4-chlorobutane (131 g, 0.767 mol) and tetrahydrofuran ( 66.1 g), and the resulting mixture was stirred at 0-5°C for 30 minutes. Then, the solution of (4E,6Z)-4,6-undecadienylmagnesium chloride prepared above in tetrahydrofuran was added dropwise at 5-15°C. After completion of the dropwise addition, the reaction mixture was stirred at 5˜10° C. for 2 hours, and then the reaction was stopped by adding ammoni...
Embodiment 3
[0237] Preparation of (9E,11Z)-1-chloro-9,11-hexadecadiene (1-2:X=Cl)
[0238] Magnesium (13.1 g, 0.540 g atom) and tetrahydrofuran (146 g) were added to the reactor, and the resulting mixture was stirred at 60-65° C. for 30 minutes. Then, (4E,6Z)-1-chloro-4,6-undecadadiene (4-1:X 1 =C1) (91.7g, 0.491mol), and then the resulting mixture was stirred at 70-75°C for 6 hours to prepare (4E,6Z)-4,6-undecadienylmagnesium chloride. In another reactor was charged cuprous iodide (0.98 g, 0.0049 mol), triethyl phosphite (1.96 g, 0.0118 mol), 1-bromo-5-chloropentane (105 g, 0.565 mol) and tetrahydrofuran ( 48.7 g), and the resulting mixture was stirred at 0-5°C for 30 minutes. Then, the solution of (4E,6Z)-4,6-undecadienylmagnesium chloride prepared above in tetrahydrofuran was added dropwise at 5-15°C. After the dropwise addition was complete, the resulting reaction mixture was stirred at 5-10° C. for 3 hours, and then stopped by adding ammonium chloride (4.63 g), 20% by weight aqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com