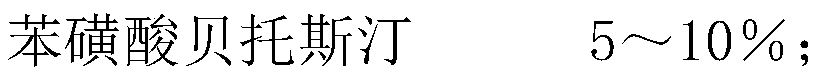Bepotastine besilate
A technology for bepotastine besylate and bepotastine besylate tablets, which are applied in the field of bepotastine besylate tablets and their preparation, can solve the problems of low dissolution rate, low bioavailability and the like, Achieve the effect of improving dissolution, simple preparation process and no solvent residue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
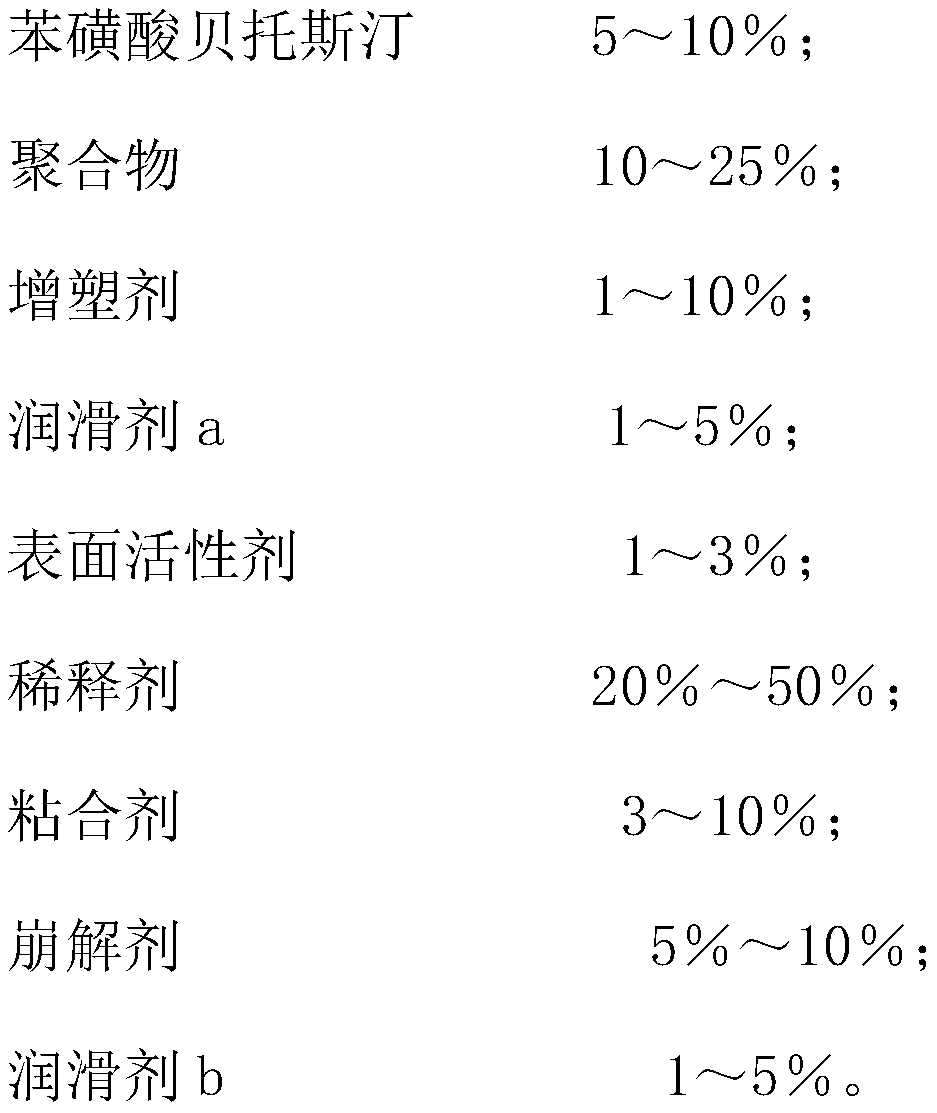
[0017] Embodiment 1, bepotastine besylate tablet components and preparation method thereof, bepotastine besylate tablet components are as follows by weight percentage,
[0018]
[0019]
[0020] Preparation method: ① Grind bepotastine besylate, crospovidone, povidone k30, mannitol, polyethylene glycol and poloxamer respectively, and pass through a 40-80 mesh sieve to carry out Mix and stir evenly; ②Set the extrusion temperature of the screw extruder at 90°C. After reaching the preset temperature, add the above-mentioned mixture into the extruder for extrusion molding, and then crush it with crushing equipment. Pass 24 mesh screening to obtain solids with uniform particle size; ③ mix the pulverized solids with microcrystalline cellulose, starch, croscarmellose sodium and magnesium stearate, and stir evenly; ④ press the mixture in step ③ Tablets are made into tablets.
Embodiment 2
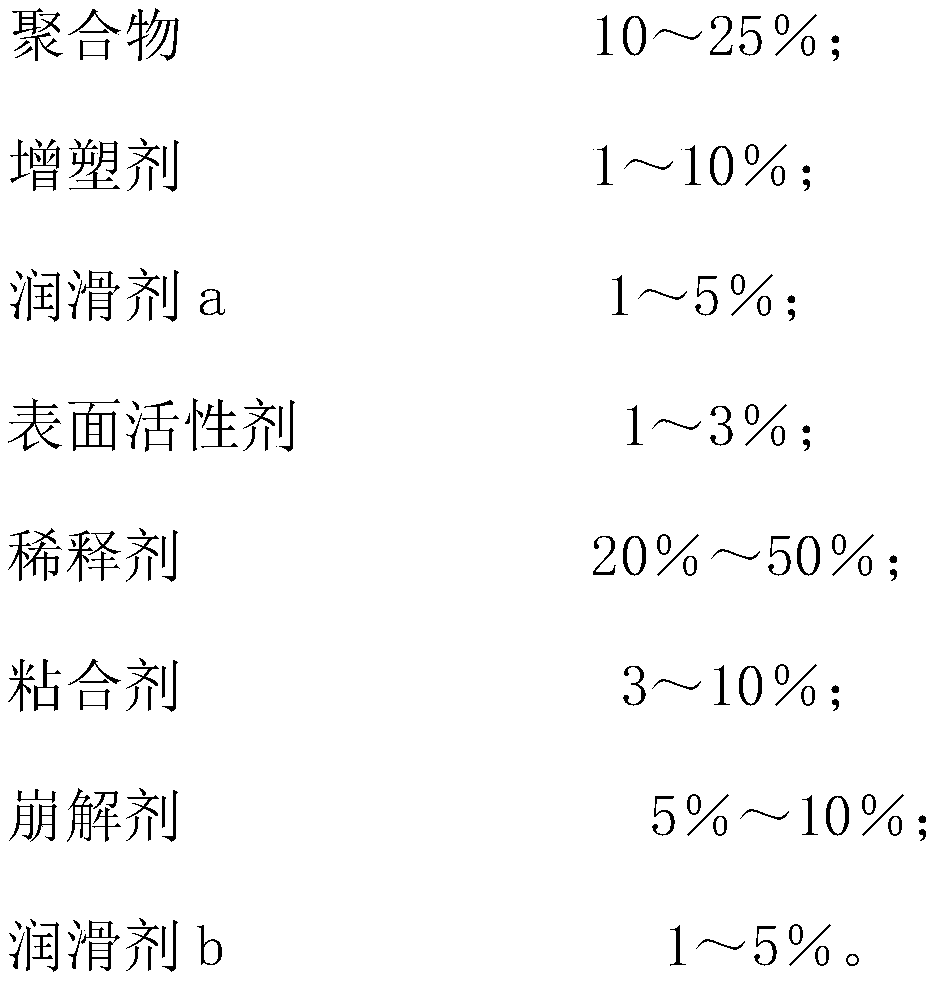
[0021] Embodiment 2, bepotastine besylate tablet components and preparation method thereof, bepotastine besylate tablet components are as follows by weight percentage,
[0022]
[0023] Preparation method: ① Grind bepotastine besylate, hydroxypropylmethylcellulose, polyethylene glycol, poloxamer and sodium lauryl sulfate respectively, and then pass through a 40-80 mesh sieve, and carry out Mix and stir evenly; ②Set the extrusion temperature of the screw extruder at 110°C. After reaching the preset temperature, add the above-mentioned mixture into the extruder for extrusion molding, and then use crushing equipment for crushing. Screening through 32 meshes to obtain a solid with uniform particle size; ③ mixing the pulverized solid with microcrystalline cellulose, lactose, croscarmellose sodium, low-substituted hydroxypropyl cellulose and magnesium stearate, and stirring evenly; ④ compress the mixture in step ③ to make tablets.
Embodiment 3
[0024] Embodiment 3, bepotastine besylate tablet components and preparation method thereof, bepotastine besylate tablet components are as follows by weight percentage,
[0025]
[0026] Preparation method: ① respectively pulverize bepotastine besilate, povidone k30, xylitol, poloxamer and glyceryl monostearate, pass through a 40-80 mesh sieve, mix, and Stir evenly; ②Set the extrusion temperature of the screw extruder at 120°C. After reaching the preset temperature, add the above-mentioned mixture into the extruder for extrusion molding, and then crush it with crushing equipment, pass through 40 mesh Screen to obtain a solid with uniform particle size; ③Mix the pulverized solid with sorbitol, sucrose, ethyl cellulose, low-substituted hydroxypropyl cellulose and magnesium stearate, and stir evenly; ④Compress the mixture in step ③ into tablets Made into tablets.
[0027] Dissolution investigation, assay method: get sample 40mg among the embodiment 1-3, according to dissolutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com