A kind of preparation method and application of CTL cells
A cell and insect cell technology, applied in the field of CTL cell preparation, can solve problems such as side effects, destroy immune tolerance, etc., and achieve the effects of high stability, improved curative effect, and high-efficiency specific cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The preparation of embodiment 1 tumor antigen PAP-GM-CSF
[0070] 1. Construction of insect baculovirus vector
[0071] (1) Gene synthesis of tumor antigen PAP-GM-CSF
[0072] The gene sequences of prostatic acid phosphatas (Prostatic Acid Phosphatas, PAP) and granulocyte-macrophage colony stimulating factor (GM-CSF) were retrieved from Genbank, and PAP and GM-CSF were passed through two The amino acid Gly-Ser connection structure contains a signal peptide upstream of PAP, and the software is used for codon optimization to synthesize the entire DNA sequence. The corresponding DNA sequence of the PAP-GM-CSF fusion protein is shown in SEQ ID NO.: 1, and the corresponding amino acid sequence is shown in SEQ ID NO.: 2.
[0073] (2) Construction of the shuttle plasmid pFast-Bac1-PAP-GM-CSF
[0074] The vector pFast-Bac1 and the gene of the synthetic tumor antigen PAP-GM-CSF were digested, connected and transformed to construct a recombinant plasmid.
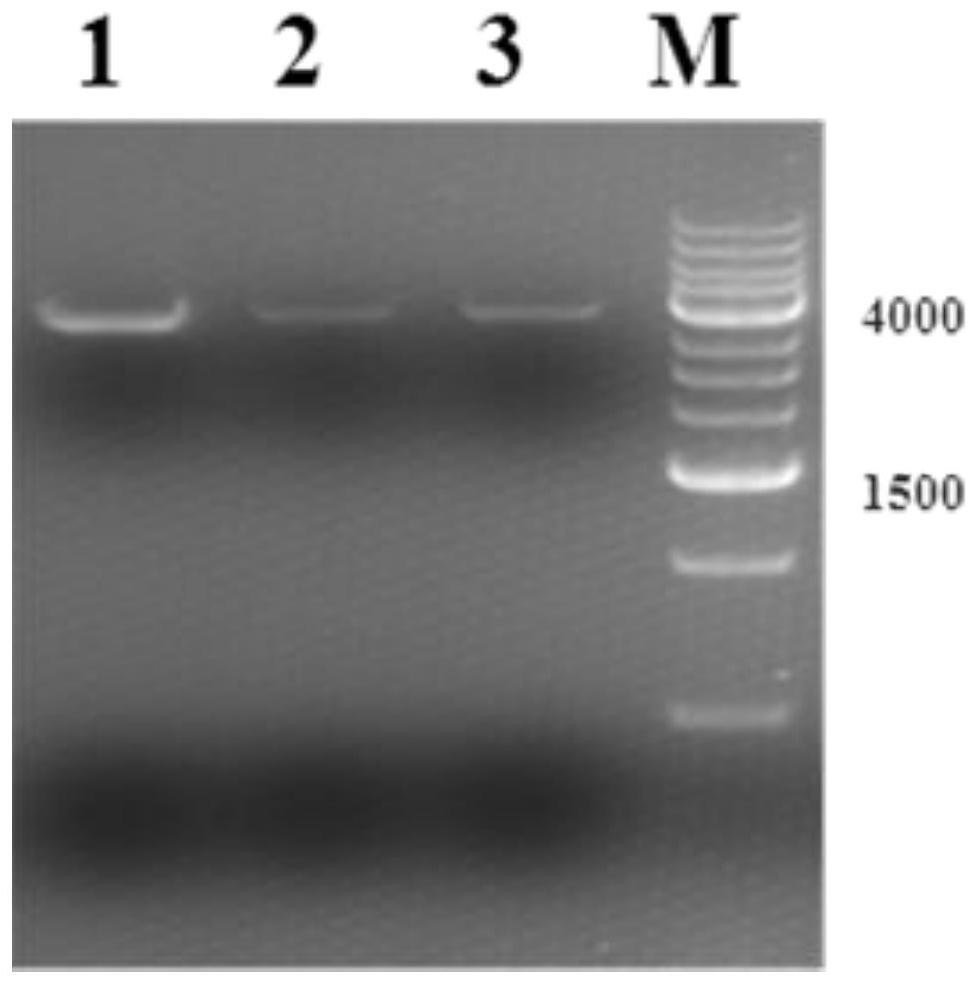
[0075] (3) Construc...
Embodiment 2
[0100] Example 2 DC cells sensitized by tumor antigen PAP-GM-CSF
[0101] Separation of peripheral blood mononuclear cells (PBMC) by a blood cell separator or Ficoll, and isolation of CD14 by magnetic bead sorting + Monocytes, add X-VIVO TM 15 (LONZA) lymphocyte serum-free medium (containing 500-1000U / ml rhGM-CSF and 500U / ml rhIL-4), 37°C, 5% CO 2 CO 2 Culture in an incubator, add tumor antigen PAP-GM-CSF on the 5th day to stimulate activated DC, add TNF-α (final concentration 20ng / ml) at the same time to induce DC maturation, continue to culture for 48h, and obtain tumor antigen PAP-GM-CSF sensitization DC cells, such as Figure 5 As shown, the proportion of CD86 of DC cells detected by flow cytometry was 54.6%.
Embodiment 3
[0102] Example 3 Sensitized DC cells induce CTL cells
[0103] Blood cell separator or Ficoll to separate PBMCs from the same source as DCs, add them to DCs stimulated and matured by PAP-GM-CSF fusion protein in Example 2 (DC:PBMCs=1:10), 37°C, 5% CO 2 co-incubated in the incubator for 3-5 days to obtain tumor antigen PAP-GM-CSF specific CTL cells, such as Image 6 shown, where Image 6 (A) Detection of CD3 in induced cells by flow cytometry + CD4 + The ratio is 19.0%, Image 6 (B) Detection of CD3 in induced cells by flow cytometry + CD8 + The proportion of 79.9%, that is, CTL cells as high as 79.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com