Method for preparing lactic acid through selective catalytic conversion of ethylene glycol
A catalytic conversion and ethylene glycol technology, applied in chemical instruments and methods, preparation of organic compounds, carboxylate preparation, etc., can solve the problems of large-scale production limitations, dilute product concentration, and easy poisoning fermentation cycle, etc., to achieve reaction High conversion efficiency and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation of embodiment 1 azacyclic carbene iridium complex 1a:
[0053]
[0054] Under nitrogen, add a stirrer and cyclooctadiene iridium chloride dimer (0.33 g) into a Schlenk tube, pump and exchange air three times, and add 10 mL each of dichloromethane and tetrahydrofuran with a syringe. After the solution was clarified, potassium tert-butoxide (0.11 g) was added, sealed and stirred at room temperature for 1 h. After the color of the solution changed from red to reddish brown, 1,3-dimethylbenzimidazole tetrafluoroborate (0.23 g) was added, sealed and stirred at room temperature for 4 h. The reaction solution was separated by column to obtain 1a. Yield: 0.47 g, 90%.
[0055] NMR analysis: 1 H NMR (400MHz, CDCl 3 )δ=7.32–7.22(m,4H),4.74(s,2H),4.17(s,6H),2.98(s,2H),2.36–2.23(m,4H),1.92–1.78(m,2H) ,1.76–1.63(m,2H); 13 C NMR (101MHz, CDCl 3 )δ=135.42, 122.53, 109.41, 86.82, 52.11, 34.29, 33.56, 29.47.
Embodiment 2
[0056] The preparation of embodiment 2 azacyclic carbene iridium complex 1b:
[0057]
[0058] Under nitrogen, the cyclooctadiene ligand product 1a (0.26 g) was dissolved in dichloromethane (10 mL), and carbon monoxide gas was continuously passed through at room temperature for 1 h. The completeness of the reaction was checked by TLC. After the reaction was complete, the solvent was concentrated to 2 mL, and enough ether was added to precipitate the product. After filtration and vacuum drying, the product 1b was obtained. Yield: 0.23 g, 95%.
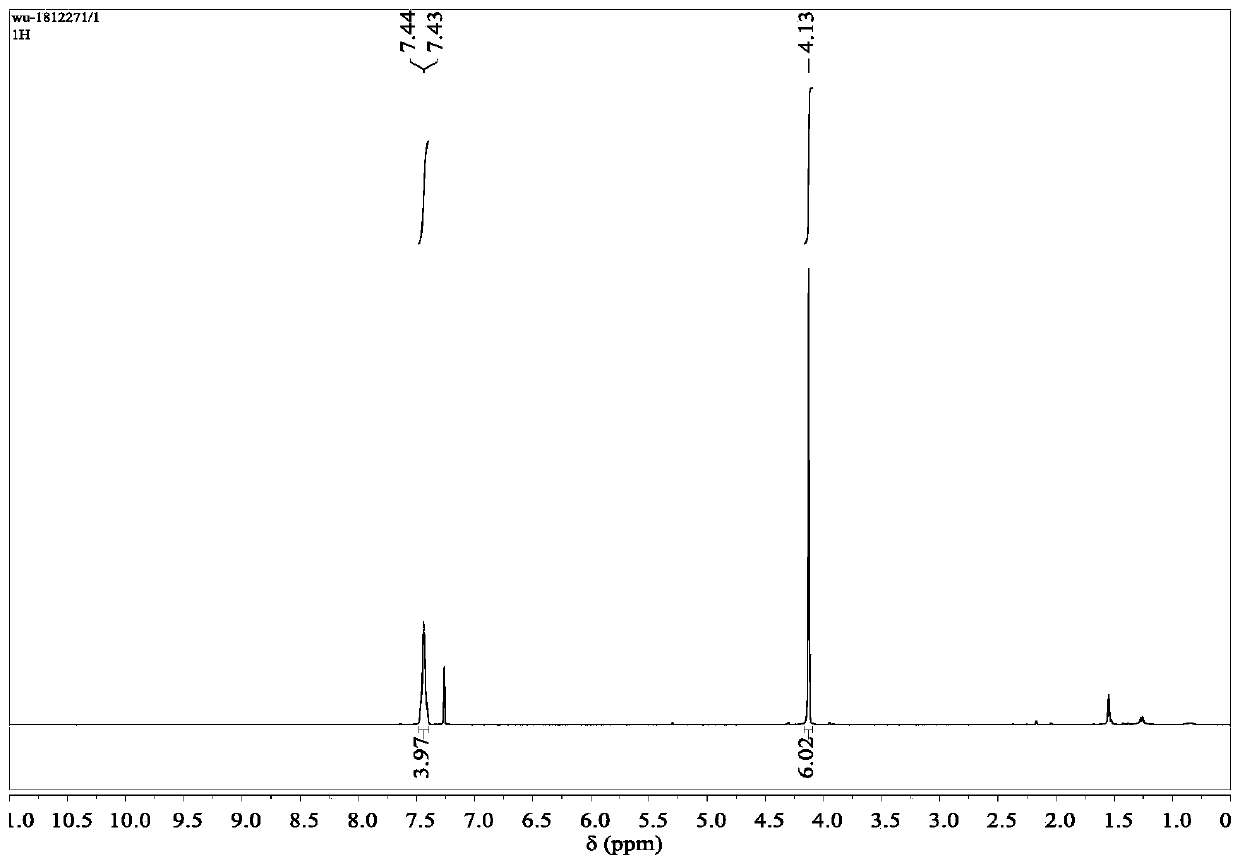
[0059] NMR analysis: 1 H NMR (400MHz, CDCl 3 )δ=7.48–7.40(m,4H),4.13(s,6H); 13 C NMR (101MHz, CDCl 3 )δ=181.27, 167.86, 134.67, 124.08, 110.68, 35.11.
Embodiment 3
[0060] The preparation of embodiment 3 azacyclic carbene iridium complex 2a:
[0061]
[0062] Under nitrogen, in a Schlenk tube, add stir bar, cyclooctadiene iridium chloride dimer (0.16 g), sodium hydride (0.05 g), 1,3-dimethylbenzimidazole tetrafluoroborate (0.23g) and dry tetrahydrofuran (15mL), sealed and stirred overnight at room temperature. The reaction solution was directly passed through the column, and the corresponding 2a was obtained after vacuum drying. Yield: 0.16 g, 48%.
[0063] NMR analysis: 1 H NMR (400MHz, DMSO-d 6 )δ=7.60–7.52(m,4H),7.35–7.27(m,4H),4.18(s,12H),4.04(s,4H),2.40(s,4H),2.08–1.98(m,4H) ; 19 F NMR (376MHz, 298K, DMSO-D 6 )δ=-148.32; 13 C NMR (101MHz, DMSO-d 6 )δ=187.87, 135.42, 123.20, 110.86, 78.18, 35.50, 31.30.
[0064] Mass spectrometry: HR-MS (ESI / TOF) m / z: Calcd.for C 26 h 32 IrN 4 [M-BF 4 ] + 593.2256; found: 593.2261.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com