Biphasic transdermal drug delivery system and preparation method thereof
A drug delivery system and drug-carrying technology, which is applied in pharmaceutical formulations, drug delivery, medical preparations of non-active ingredients, etc., can solve the problems of inapplicable temperature-sensitive drugs, active drugs prone to inactivation, and uncontrollable release rates, etc. problems, achieve good skin affinity and transdermal performance, good skin compatibility, and improve solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
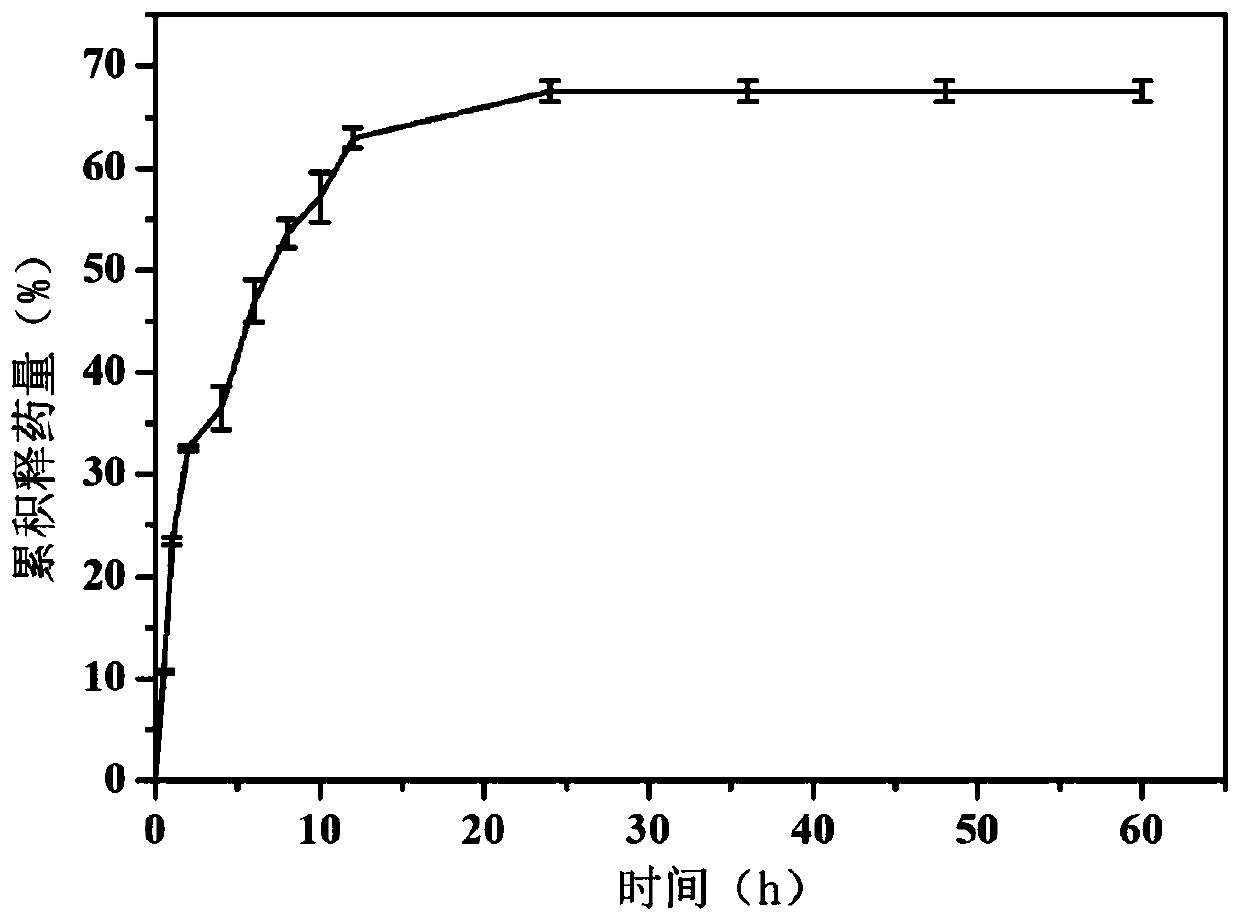
[0030] (1) Configure silk fibroin spinning solution (solvent is water) containing 20% silk fibroin (mass volume ratio) and 2% PEO (mass volume ratio), and add drug-loaded alcohol according to the volume ratio of 40% Plastid, medicine is doxorubicin hydrochloride, concentration is 1mg / mL (mass volume ratio);
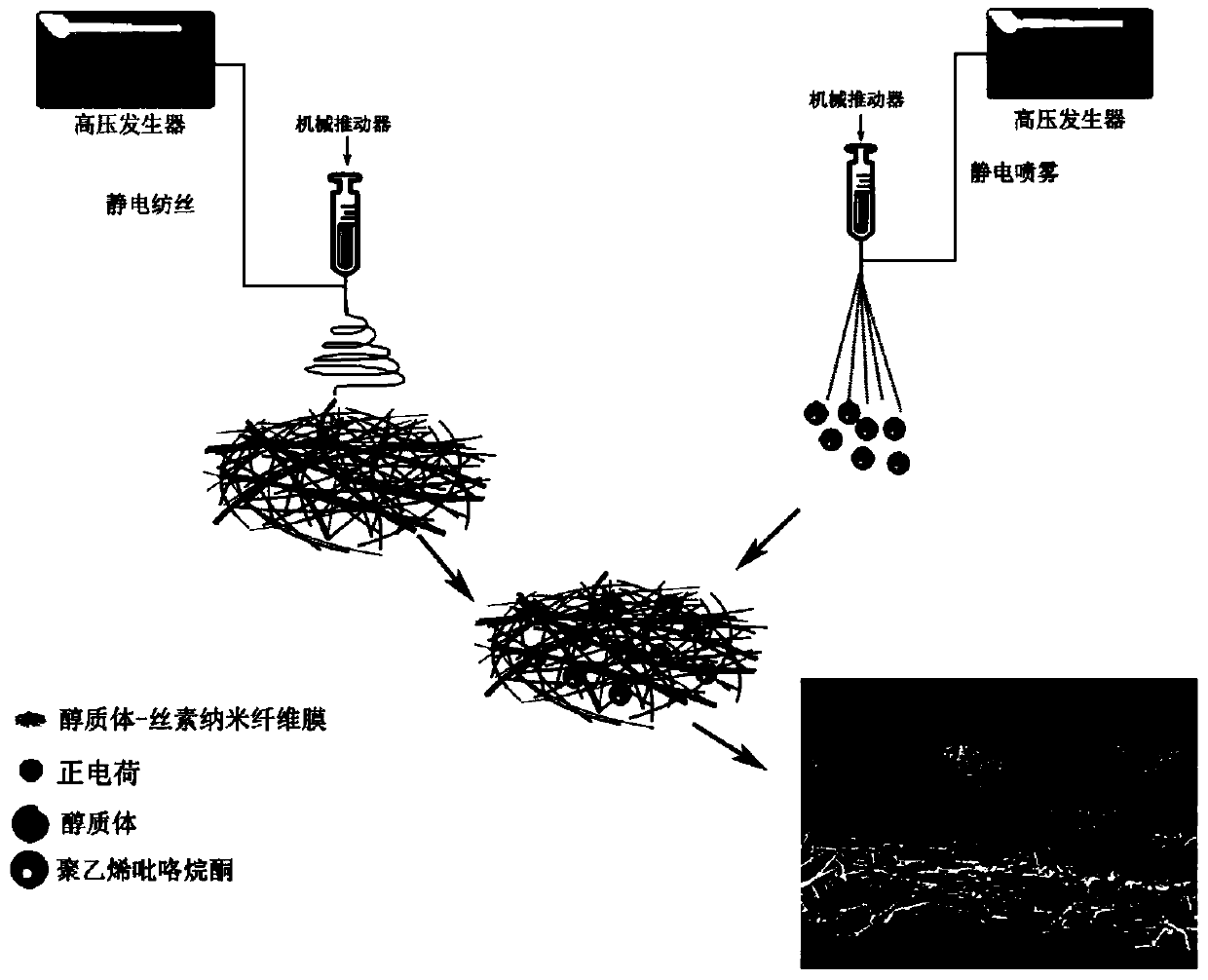
[0031] (2) Transfer the spinning solution in step (1) to the syringe, and set the electrospinning parameters: the voltage is 12kV, the advancing speed is 1mL / h, the receiving distance is 15cm, the temperature is 25°C, and the humidity is 10%. The receiver receives and prepares the drug-loaded ethosome-silk fibroin nanofiber membrane;
[0032] (3) placing the nanofibrous membrane prepared in step (2) in a fumigator filled with 75% ethanol for crosslinking for 24 hours;
[0033] (4) Configure 10% polyvinylpyrrolidone K30 (mass volume ratio) electrospray solution (solvent is 1-10:10-100 ethanol aqueous solution by mass ratio), and add drug-loaded alcohol in a ratio of 40%...
Embodiment 2
[0041] (1) Configure a collagen spinning solution (solvent is water) containing 20% collagen (mass volume ratio) and 2% PEO (mass volume ratio), and add drug-loaded ethosomes according to the volume ratio of 40% , the medicine is ketoprofen, and the concentration is 1mg / mL (mass volume ratio);
[0042] (2) Transfer the spinning solution in step (1) to the syringe, and set the electrospinning parameters: the voltage is 15kV, the advancing speed is 0.5mL / h, the receiving distance is 10cm, the temperature is 30°C, and the humidity is 5%. Received by the receiver to prepare the drug-loaded ethosome-silk fibroin nanofiber membrane;
[0043] (3) placing the nanofibrous membrane prepared in step (2) in a fumigator filled with 75% ethanol for crosslinking for 24 hours;
[0044] (4) Configure 10% polyvinylpyrrolidone k30 (mass volume ratio) electrospray solution (solvent is 1-10:10-100 ethanol aqueous solution by mass ratio), and add drug-loaded alcohol in a ratio of 40% by volume ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com