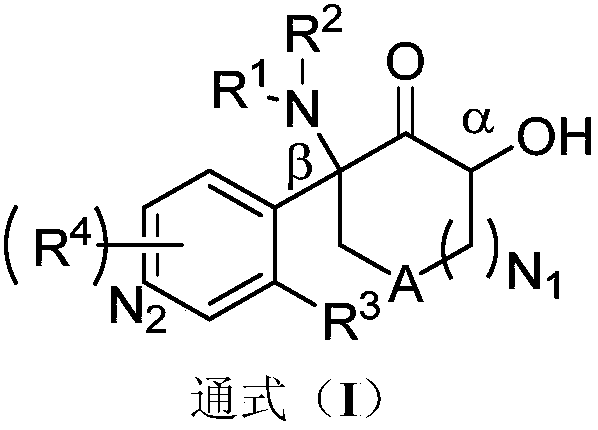
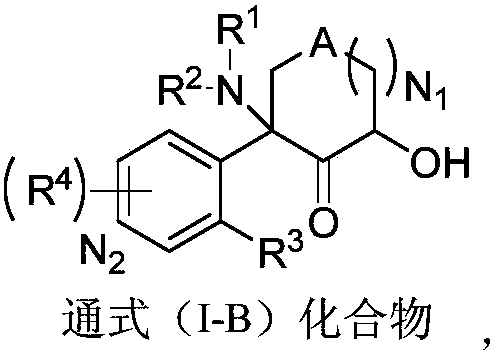
Aromatic compound, preparation method and use of aromatic compound
A technology for compounds and mixtures, applied in the field of treatment and/or prevention of depression-related diseases, novel aromatic compounds and their preparation, can solve the problems of poor drugability of compounds, low central nervous system dosage, difficult drugs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Example 1: Preparation of 2-amino-6-hydroxyl-2-o-tolylcyclohexane-1-one (compound 1)
[0150]
[0151] Step a: Preparation of 1-a
[0152]
[0153] 2-Bromotoluene (13g, 76mmol) was dissolved in dry THF (120mL), protected by Ar, cooled to -90°C with liquid nitrogen ethanol, and n-BuLi (31.92mL, 79.8mmol) was added dropwise for 30 minutes. After stirring at -90°C for 30 min, epoxycyclohexane (8.49 mL, 83.6 mmol) and boron trifluoride ether solution (10.55 mL, 83.6 mmol) were added dropwise, and the mixture was stirred in liquid nitrogen ethanol for 1.5 hours. The reaction was monitored by TLC (EA:PE=1:5), and after the reaction was completed, the saturated NH4Cl solution (100 mL) was used to quench the reaction, and H 2 Diluted with O (150ml), extracted with ethyl acetate (150mL×3), combined the organic phases, washed with saturated NaCl solution, dried over anhydrous sodium sulfate, filtered, and the filtrate was rotary evaporated to remove the low boiling solvent...
Embodiment 2
[0174] Example 2: Preparation of 2-amino-6-hydroxyl-2-m-tolylcyclohexane-1-one (compound 2)
[0175]
[0176] Step a: Preparation of 2-a
[0177]
[0178] Using m-bromotoluene (13.2g, 77.17mmol) and epoxycyclohexane (8.3g, 84.56mmol) as raw materials, prepared according to the method described in step a in Example 1, to obtain 10.95g of yellow oily liquid, yield: 74.6%. 1 H NMR (400MHz, CDCl 3 )δ7.23(t, J=7.4Hz, 1H), 7.06(d, J=7.9Hz, 3H), 3.71–3.60(m, 1H), 2.41(dd, J=12.3, 3.3Hz, 1H), 2.36(d, J=5.3Hz, 3H), 2.12(dd, J=8.1, 3.6Hz, 1H), 1.90–1.81(m, 2H), 1.80–1.72(m, 1H), 1.47–1.30(m, 4H).MS(M+Na) + : 213.1
[0179] Step b: Preparation of 2-b
[0180]
[0181] Using compound 2-a (1.53 g, 8.04 mmol) as raw material, it was prepared according to the method described in step b of Example 1 to obtain 1.33 g of yellow oily liquid, yield: 88.1%. 1 H NMR (400MHz, CDCl 3 )δ7.24(t, J=7.5Hz, 1H), 7.08(d, J=7.4Hz, 1H), 6.95(d, J=7.7Hz, 2H), 3.58(dd, J=12.1, 5.4Hz, 1H),2.56...
Embodiment 3
[0200] Example 3: Preparation of 2-amino-6-hydroxyl-2-m-fluorophenylcyclohexane-1-one (compound 3)
[0201]
[0202] Step a: Preparation of 3-a
[0203]
[0204] Taking m-fluorobromobenzene (9.63g, 55.03mmol) and epoxycyclohexane (5.94g, 60.52mmol) as raw materials, prepared according to the method described in step a in Example 1, to obtain 8.21g of yellow oily liquid, the yield : 76.8%. 1 H NMR (400MHz, CDCl 3 )δ7.32–7.27(m,1H),7.03(d,J=7.5Hz,1H),6.94(dd,J=16.8,9.1Hz,2H),3.64(td,J=9.9,4.2Hz,1H ),2.49–2.38(m,1H),2.12(d,J=8.0Hz,1H),1.86(d,J=10.2Hz,2H),1.77(d,J=12.2Hz,1H),1.48–1.32 (m,4H).MS(M+Na) + :217
[0205] Step b: Preparation of 3-b
[0206]
[0207] Using compound 3-a (1.829 g, 9.42 mmol) as raw material, it was prepared according to the method described in step b of Example 1 to obtain 1.303 g of yellow oily liquid, yield: 72.0%. 1 H NMR (400MHz, CDCl 3 )δ7.32–7.27(m,1H),6.94(ddd,J=18.4,7.3,4.8Hz,2H),6.88–6.83(m,1H),3.61(dd,J=12.1,5.4Hz,1H) ,2.57–2.50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com