Use of phosphodiesterase 9A inhibitor for preventing and treating vascular dementia
A technology of phosphodiesterase and vascular dementia, applied in the field of medicine, can solve problems such as LW33 vascular dementia that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
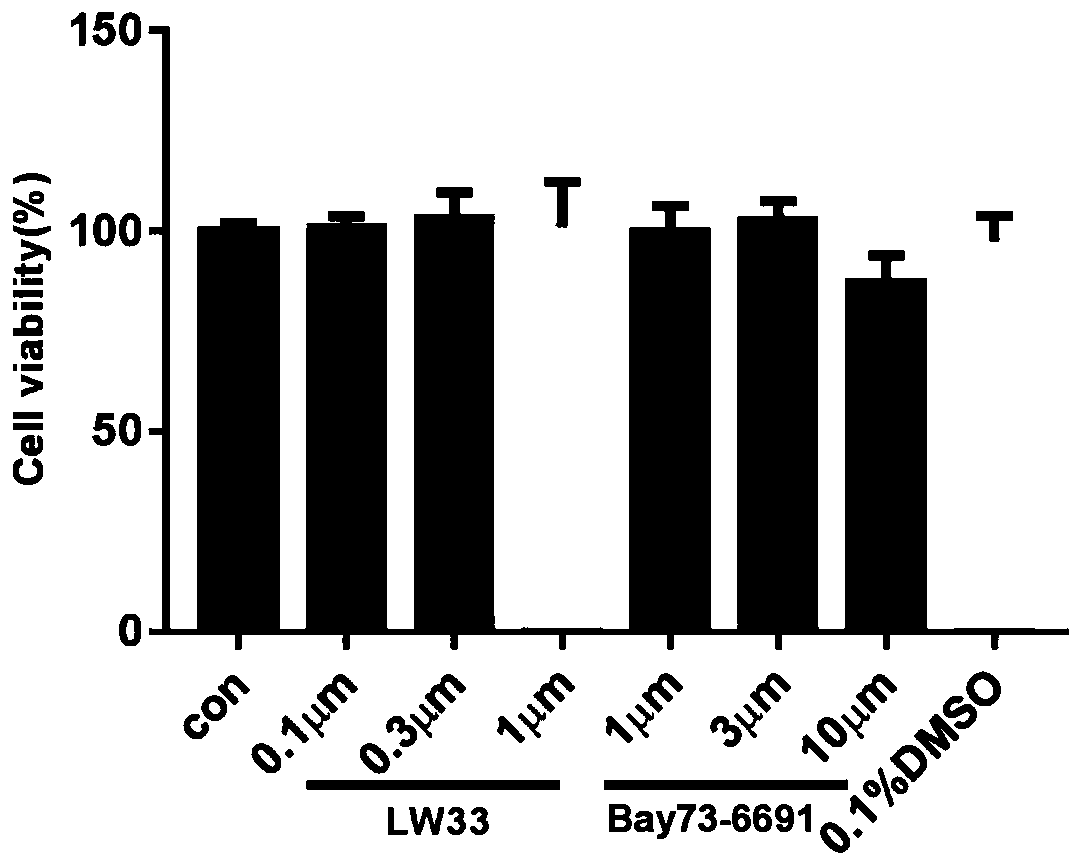
[0033] Embodiment 1, the impact of LW33 on the viability of BV2 cells
[0034](1) Experimental materials: BV2 (mouse microglia), purchased from the Cell Bank of the Chinese Academy of Sciences; DMEM medium (Gibco, USA); fetal bovine serum (Gibco, USA); CCK8 (Dojin Institute of Chemistry, Japan); Bay73- 6691(1-(2-chlorophenyl)-6-[(2R)-3,3,3-trifluoro-2-methylpropyl]-1,5-dihydro-4H-pyrazolo[3 ,4-d]pyrimidin-4-one, Bayer); PF-04447943 (6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl] -1-(tetrahydro-2H-pyran-4-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one, MCE, USA).
[0035] (2) Experiment method: 5.0×10 3 The BV2 cells were added to the wells of a 96-well plate and cultured for 24 hours. The original culture solution was aspirated, and the complete culture solution containing LW33 at different concentrations (0.1 μM, 0.3 μM, 1 μM) was added, and the complete culture solution without LW33 was added as a normal control. Continue culturing for 24 hours, add 10 μ...
Embodiment 2
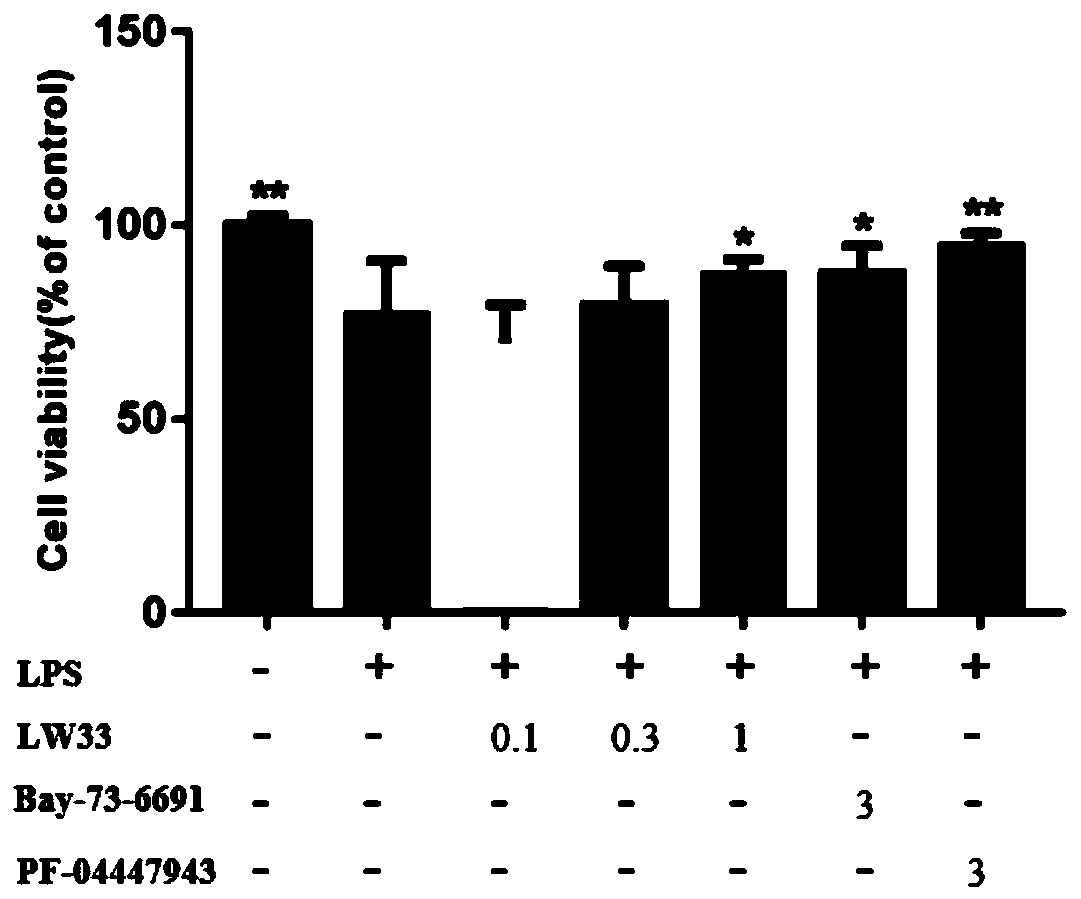
[0037] Example 2, the effect of LW33 on the proliferation of BV2 cells induced by LPS
[0038] (1) Experimental materials
[0039] Mouse microglia BV2 (Cell Bank, Chinese Academy of Sciences), DMEM medium (Gibco, USA), fetal bovine serum (Gibco, USA), CCK8 (Japanese colleagues), LPS (Sigma)
[0040] (2) Experimental method
[0041] 5.0×10 3 The BV2 cells were added to the wells of the 96-well plate and cultured for 24 hours. Divide BV2 cells into:
[0042] Control group: LPS treatment group (1μg / mL action time 24h);
[0043] LPS treatment group (1μg / mL action time 24h)+LW33 0.1μM;
[0044] LPS treatment group (1μg / mL action time 24h)+LW33 0.3μM;
[0045] LPS treatment group (1μg / mL action time 24h)+LW33 1μM;
[0046] LPS treatment group (1μg / mL action time 24h)+Bay73-6691 3μM;
[0047] LPS treatment group (1μg / mL action time 24h)+PF-04447943 3μM;
[0048] After 24h, CCK8 detected cell proliferation. The LPS unstimulated group was used as the control, and the differen...
Embodiment 3
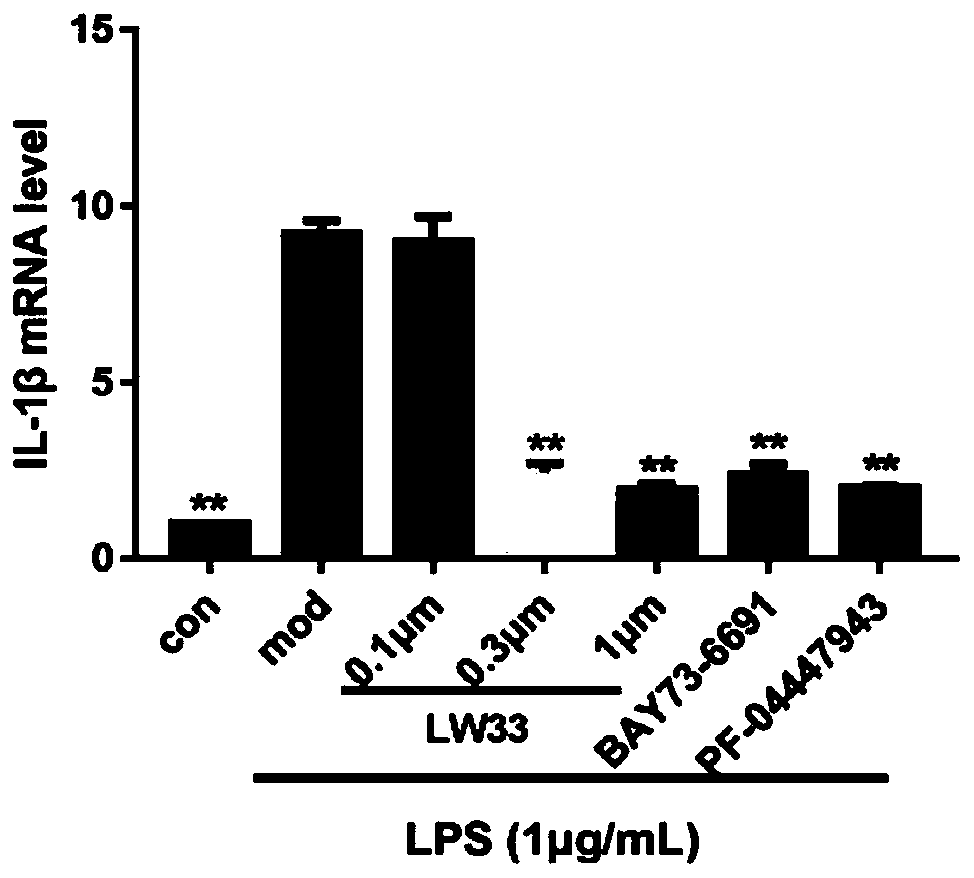
[0050] Example 3. Effect of LW33 on LPS-induced BV2 inflammatory factor mRNA level
[0051] (1) Materials and methods: HLF cells were purchased from the Experimental Animal Center of Sun Yat-sen University;
[0052] BV2 was purchased from the Cell Bank of the Chinese Academy of Sciences, DMEM medium (Gibco, USA), fetal bovine serum (Gibco, USA), 0.25% trypsin (Gibco, USA), double antibody (Gibco, USA), LPS (Sigma), DMSO (MPBIO), IL-1, IL-1β, TNF-α primers (Shanghai Sangong), Thermo Revert Aid Kit kit (Thermo Fisher Scientific)
[0053] (2) Test method
[0054] The cells were seeded in 60mm dishes and cultured in a cell culture incubator. When the cells grew to 50-60% confluent, BV2 cells were pretreated with 0.1, 0.3, 1 μM LW33 and 3 μM Bay73-6691, PF-04447943 for 1 hour, then added 1 μg / mL LPS for stimulation, and extracted after 24 hours The total cellular RNA was subjected to real-time fluorescence quantitative PCR test. After measuring the concentration, take 1 μg of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com