Separation and purification method of blood coagulation factor xiii and its application
A coagulation factor, separation and purification technology, applied in separation methods, peptide preparation methods, chemical instruments and methods, etc., can solve the problems of consuming a large amount of human blood, low extraction efficiency, low yield, etc., to avoid the reduction of protein activity, Improve extraction efficiency and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
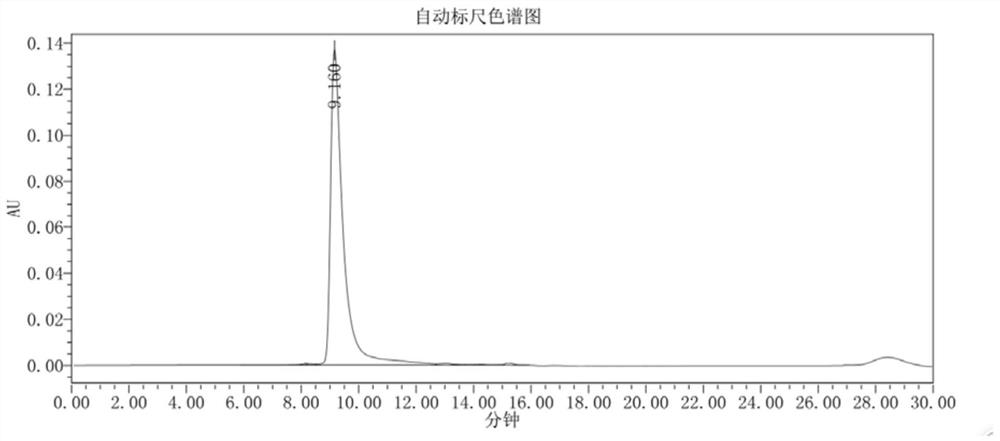
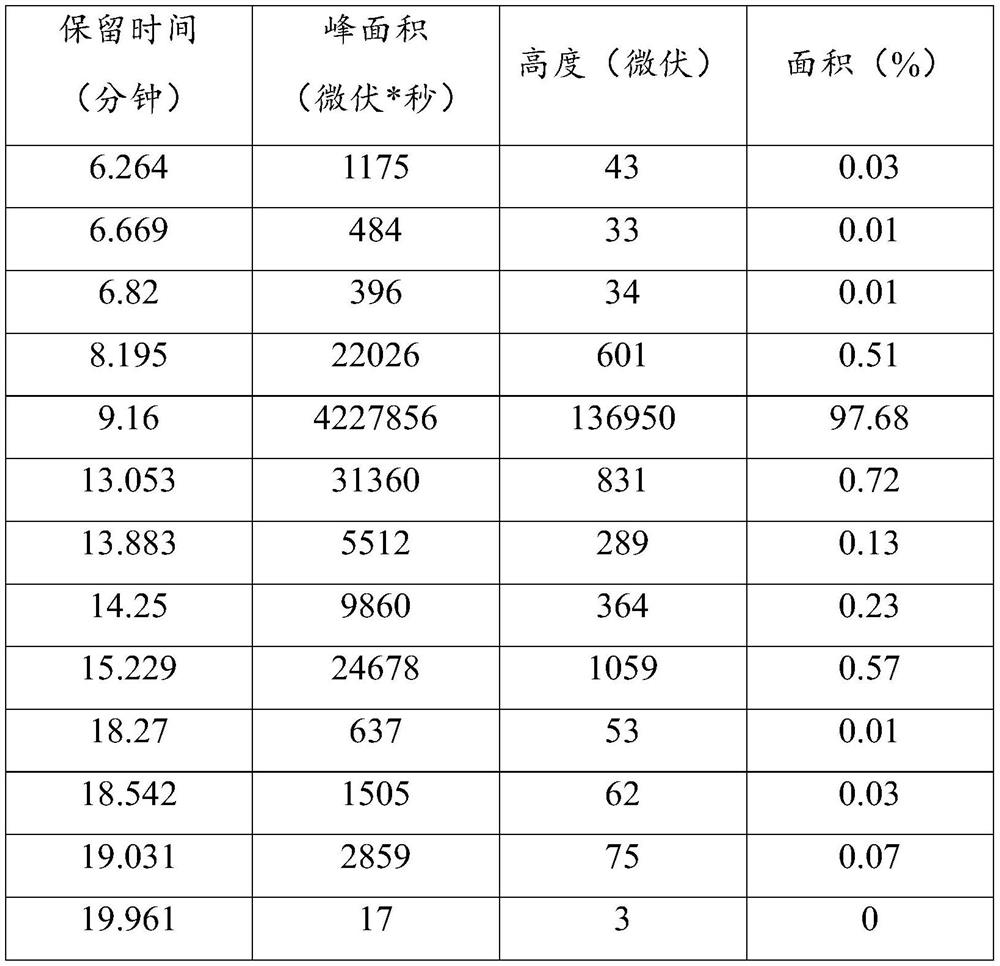
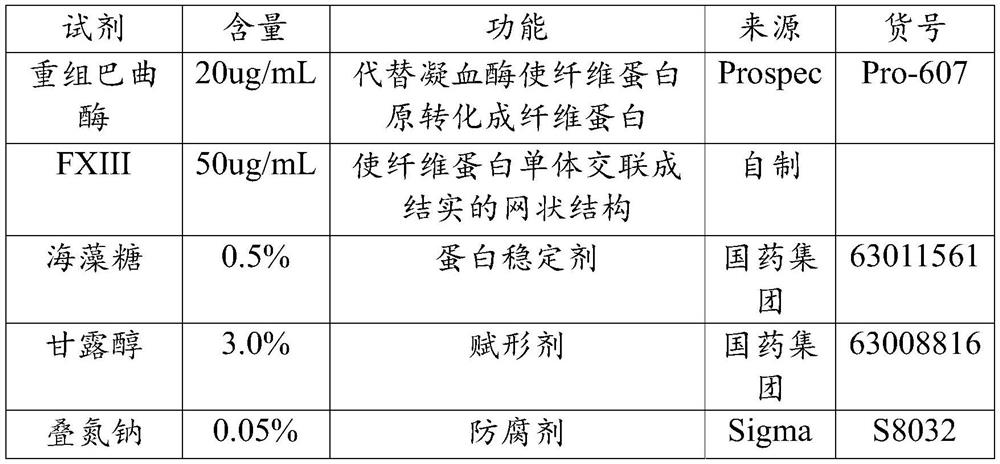
Image
Examples
preparation example Construction
[0043] As a preferred embodiment, the immunized animals of the immune response are BalB / C mice. BalB / C mice can rapidly induce plasmacytoma after injection of mineral oil, and this strain is widely used in the production of hybridoma and monoclonal antibody. The use of BALB / c mice in this protocol improves the efficiency and cost of monoclonal antibody production. In a preferred embodiment of the present invention, the preparation method of the active component containing blood coagulation factor XIII comprises the following steps:
[0044] Firstly, mammalian plasma is separated and subjected to membrane filtration to obtain component A; then component A is prepared as cryoprecipitate and then resuspended, and the supernatant is collected by solid-liquid separation to obtain the active component containing coagulation factor XIII.
[0045] As a preferred embodiment, the above-mentioned active components containing coagulation factor XIII are extracted by using mammalian blood...
Embodiment 1
[0072] Embodiment 1 prepares the active component containing coagulation factor XIII as antigen from pig blood
[0073] The preparation method of the active component containing blood coagulation factor XIII comprises the following steps:
[0074] (a) Weigh 20 kg of fresh citrated anticoagulated porcine whole blood, centrifuge at 8,000 rpm at 4°C for 20 min, collect the upper layer of plasma, and obtain component 1;
[0075] (b), component 1 is filtered with a 0.45um microporous membrane to obtain component 2;
[0076] (c) Put component 2 in a freezer at -40°C overnight to freeze all the plasma, then take it out and put it in a freezer at 4°C to slowly thaw, during the thawing process, a large amount of cryoprecipitate will be precipitated. Centrifuge at 10,000 g for 30 min in a GL-21M centrifuge to collect cryoprecipitate at 4°C to obtain fraction 3;
[0077] (d) Resuspend the cryoprecipitate of component 3 with 1000mL 8% cold ethanol solution, centrifuge at -3°C to collect...
Embodiment 2
[0083] Example 2 Preparation of Mouse Monoclonal Antibody
[0084] (1) Immunization of Balb / C mice
[0085] Take 5 Balb / C mice, immunize for the first time on the first day, mix the antigen + the same amount of complete Freund's adjuvant, fully emulsify, 100μg / mouse, and inject subcutaneously. The total amount of injection is 200 μl. The second immunization was carried out on the 14th day, and the antigen + equal amount of incomplete Freund's adjuvant was mixed, fully emulsified, 100 μg per mouse, and injected subcutaneously.
[0086] One week later, measure the potency by ELISA method. If the titer does not meet the fusion requirements, immunize once every 14 days according to the second immunization method, and measure the titer one week later. Three days after the last booster immunization, the animals were sacrificed by removing their eyeballs and bleeding, and the blood was stored for later use.
[0087] (2) Fusion cell preparation
[0088] Aseptically remove the sple...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com