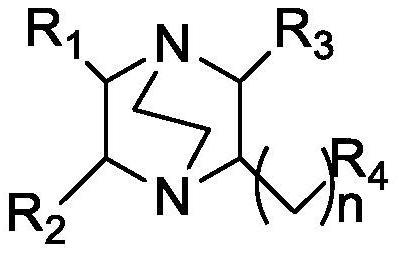
A kind of azabicyclic complex catalyst and its preparation method and application
A technology of azabicyclic and complexes, which is applied in the field of azabicyclic tertiary amine complex catalysts and its preparation, and can solve problems such as environmental pollution, low yield, and uneconomical synthesis reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0076] Example 1-6 Preparation of azabicyclic tertiary amine complex catalyst
[0077] Examples 1-6 prepared the azabicyclic tertiary amine complex catalyst of the present invention by the method of the present application, the yields of the azabicyclic tertiary amine complexes were all above 85%, and Examples 1 and 3 used The mother liquor of the previous batch was used as the reaction solvent, and the yield reached more than 95%.
Embodiment 1
[0078] Example 1: Preparation of the complex of formula I-2 by using cuprous bromide and the previous batch of mother liquor
[0079] (1) Pretreatment of cuprous bromide
[0080] The cuprous bromide produced by industrial production or purchased by the reagent company has a trace amount of cupric bromide, and the color is light green. In order to ensure the catalytic effect, pretreatment is required.
[0081] Add 450g of 3% diluted HBr to the four-necked bottle, add 0.5g Na 2 SO 3 , remove the oxidized bromine or oxygen in dilute HBr, and at the same time reduce cupric ions to cuprous ions, quickly add 1000g of reagent grade CuBr, replace it with high-purity nitrogen three times, and stir at room temperature for about 2h, Since CuBr is insoluble in dilute HBr, CuBr 2 Dissolved in dilute HBr, in order to achieve the purpose of separation and purification, filter under nitrogen protection, rinse twice with absolute ethanol to remove water, and vacuum dry at 50-60 ° C for 2 ...
Embodiment 2
[0086] Example 2: Preparation of complexes of formula I-2 using cuprous bromide
[0087] (1) the pretreatment method of cuprous bromide is with embodiment 1
[0088] (2) Preparation of formula I-2
[0089] Measure 400 mL of anhydrous acetonitrile, start stirring, then add 287.48 g of cuprous bromide (99.7%) powder, under nitrogen protection, heat to 45-50° C. to dissolve, it is a light gray solution, ready for use.
[0090] In a four-necked flask, add 126.20g of 2-methyltriethylenediamine, add 200mL of acetonitrile, dissolve at room temperature, fill with nitrogen protection, and use a constant pressure addition funnel to drop the cuprous bromide solution prepared above into , the temperature is 20-30 ℃, after adding for about 20 minutes, light brown ligand compounds are continuously precipitated. After adding, stirring at room temperature for 1 hour, then cooling to -10 ℃, crystallization, nitrogen protection suction filtration, to avoid inhalation of water, and then use ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com