Preparation of insoluble drug sustained-release film
A technology for insoluble drugs and insoluble drugs, applied in drug delivery, medical preparations containing active ingredients, pharmaceutical formulas, etc., to achieve the effect of simple process, low cost and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
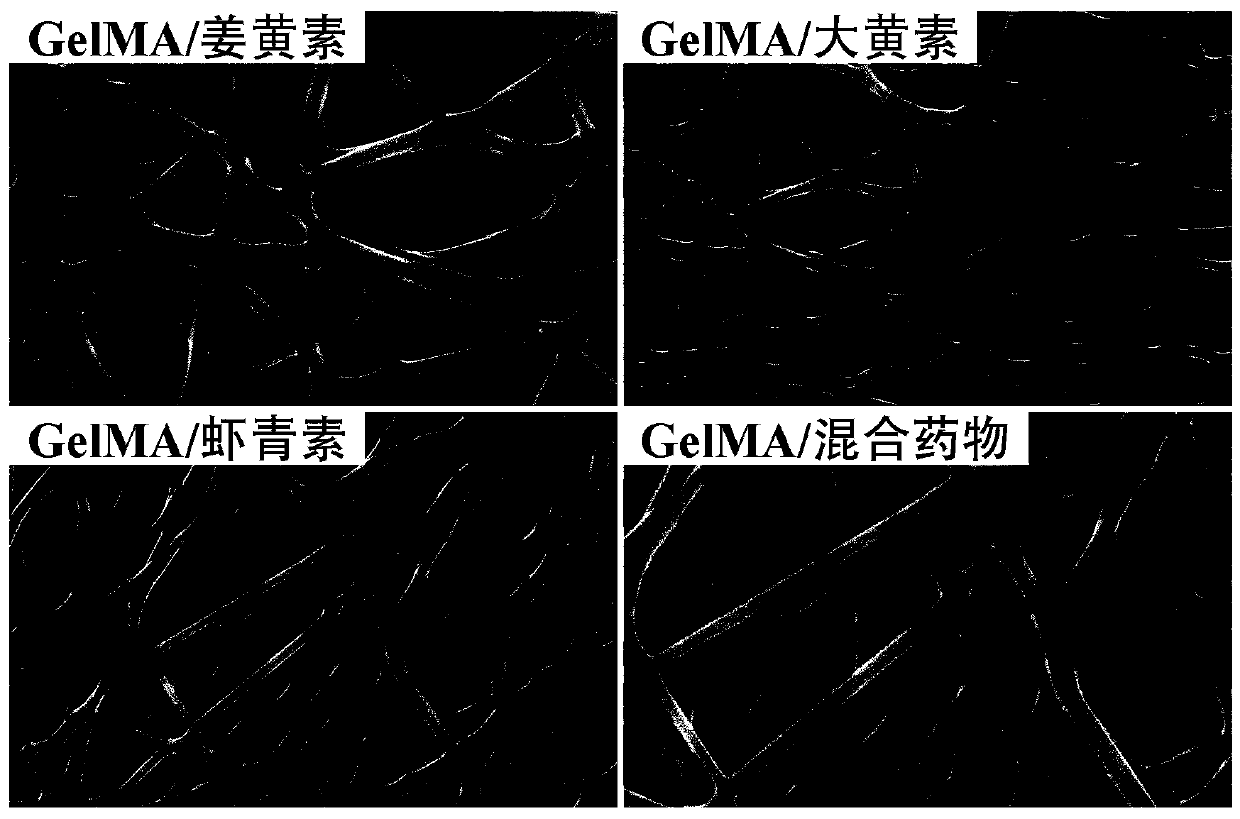
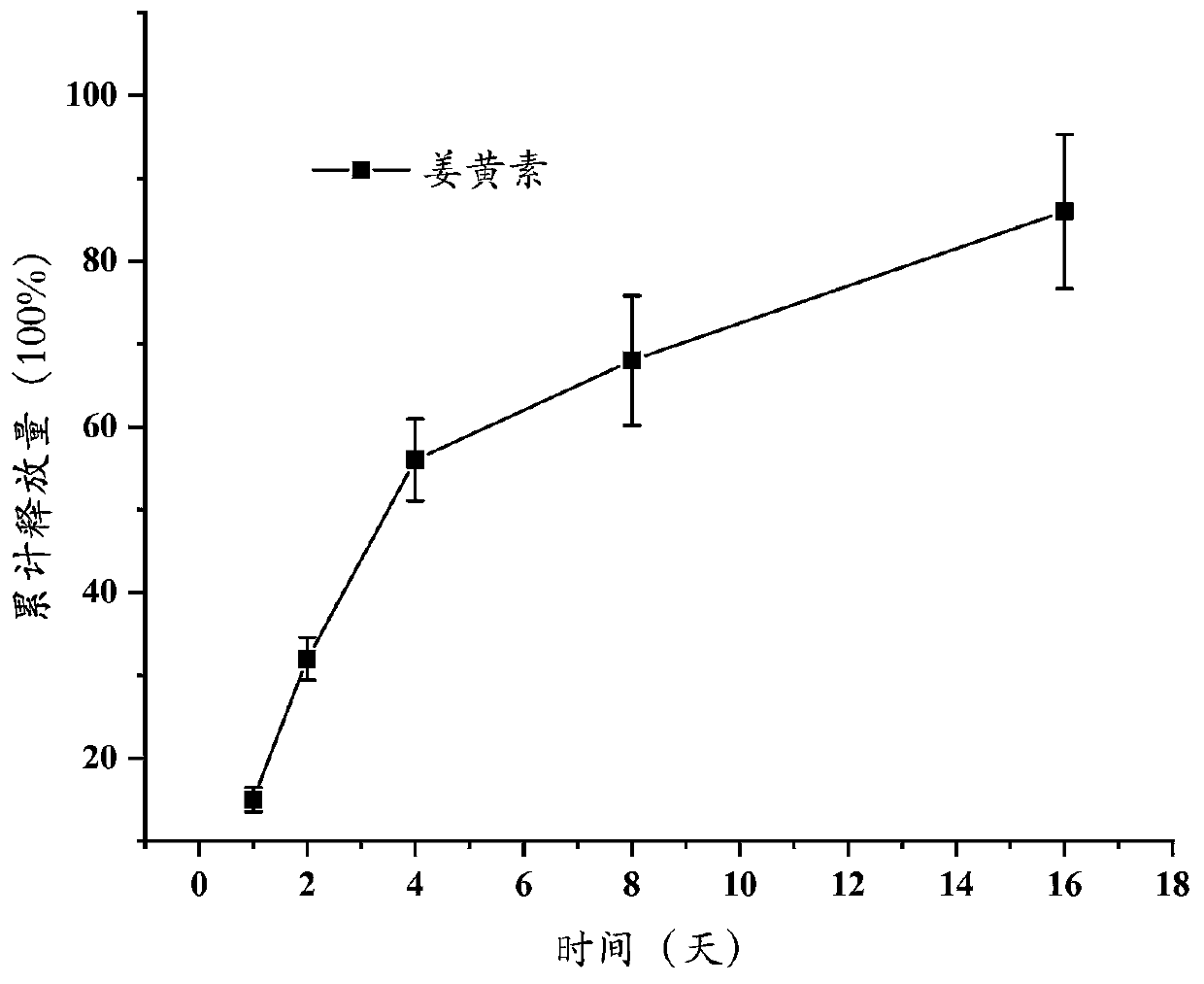
[0026] Weigh 1 g of GelMA (substitution degree is 80%), dissolve it in 10 mL of trifluoroethanol, rotate and shake at a speed of 300 rpm on a shaker at 37 °C for 8 hours, take it out, and obtain a clear and transparent solution ; Add 0.1 g of curcumin, and continue to rotate and shake at a speed of 100 rpm on a shaker at 37 ° C for 2 hours to obtain a drug-loaded mixed spinning solution.
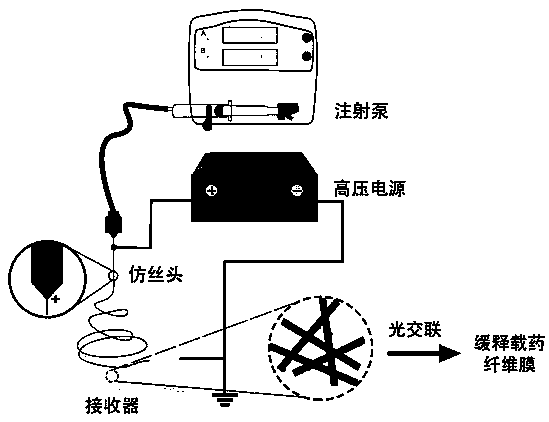
[0027] Pour the prepared spinning solution into the liquid feeder (5 mL syringe), use a flattened 25 G injection needle as the injection tube, connect the negative electrode of the high-voltage power supply, connect the aluminum foil fiber receiving plate to the positive electrode, and control the injection volume of the solution by the syringe pump . Turn on the power, adjust the speed of the syringe pump to 1.5 mL / h, adjust the receiving distance to 15 cm, then start the high-voltage power supply, and set the voltage to 15 kV. The GelMA / curcumin nanofiber membrane can be collected. Subse...
Embodiment 2
[0029] Weigh 1 g of GelMA, dissolve it in 10 mL of trifluoroethanol solution, shake it on a shaker at 37 °C at a speed of 300 rpm for 4 hours, take it out, and obtain a clear and transparent solution; then add 0.1 g of rhubarb The element was rotated and shaken at 37°C at a speed of 100 rpm for 2 hours on a shaker to obtain a mixed solution.
[0030] Pour the prepared spinning solution into the liquid feeder (5 mL syringe), use a flattened 25 G injection needle as the injection tube, connect the negative electrode of the high-voltage power supply, connect the aluminum foil fiber receiving plate to the positive electrode, and control the injection volume of the solution by the syringe pump . Turn on the power supply, adjust the speed of the syringe pump to 1 mL / h, adjust the receiving distance to 10 cm, then start the high-voltage power supply, and set the voltage to 13 kV. The GelMA / emodin nanofiber membrane can be collected. Subsequently, the collected membranes were placed...
Embodiment 3
[0032] Weigh 1.5 g of GelMA, dissolve it in 10 mL of trifluoroethanol solution, rotate and shake at a speed of 350 rpm on a shaker at 30 °C for 4 hours, take it out, and obtain a clear and transparent solution; then add 0.25 g of emodin , on a shaker at 30°C at a speed of 100 rpm for 2 hours to obtain a mixed solution.
[0033] Pour the prepared spinning solution into the liquid feeder (5 mL syringe), use a flattened 25G injection needle as the injection tube, connect the negative electrode of the high-voltage power supply, connect the aluminum foil fiber receiving plate to the positive electrode, and control the injection volume of the solution by the syringe pump. Turn on the power supply, adjust the speed of the syringe pump to 6 ml / h, adjust the receiving distance to 20 cm, then start the high voltage power supply, and set the voltage to 18kV. The GelMA / astaxanthin nanofiber membrane can be collected. Subsequently, the collected membranes were placed in a crosslinker solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com