Preparation method of roxadustat
A compound and selected technology, applied in organic chemistry, bulk chemical production, etc., can solve problems such as excessive three wastes, production existence, and separation difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
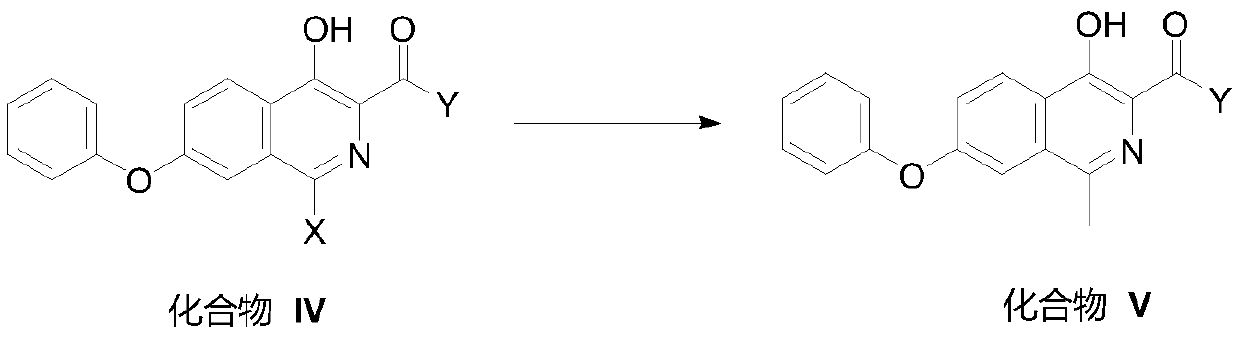
[0166] Compound I-1 produces compound II-1
[0167]
[0168] Into a 100mL three-necked flask, add 30mL of water and 2.4g of sodium hydroxide, stir to dissolve, then add 30mL of methanol and 5.90mL of methyl 4-hydroxy-7-phenoxyisoquinoline-3-carboxylate (compound I-1) g, incompletely soluble. Heat to reflux for 8h, then lower to 0-10°C, add 1N hydrochloric acid to adjust pH=2-3, stir for 0.5h. After suction filtration, the filter cake was washed with 20 mL of water and 20 mL of methanol, and dried in vacuum to obtain 5.04 g of a white solid. Yield: 90%. MS(+): m / z 282.26(M+1); NMR data ( 1H NMR, TFA-d, 400MHz): δppm 9.035 (1H, s, CH), 8.838-8.861 (1H, d, J = 9.2Hz, CH), 8.153-8.182 (1H, dd, J = 2.4Hz, CH ),7.729-7.734(1H,d,J=2.0Hz,CH),7.601-7.640(2H,t,J=8Hz,CH),7.459-7.495(1H,t,J=7.2Hz,CH),7.269 -7.289 (2H,t,J=8Hz,CH).
Embodiment 2
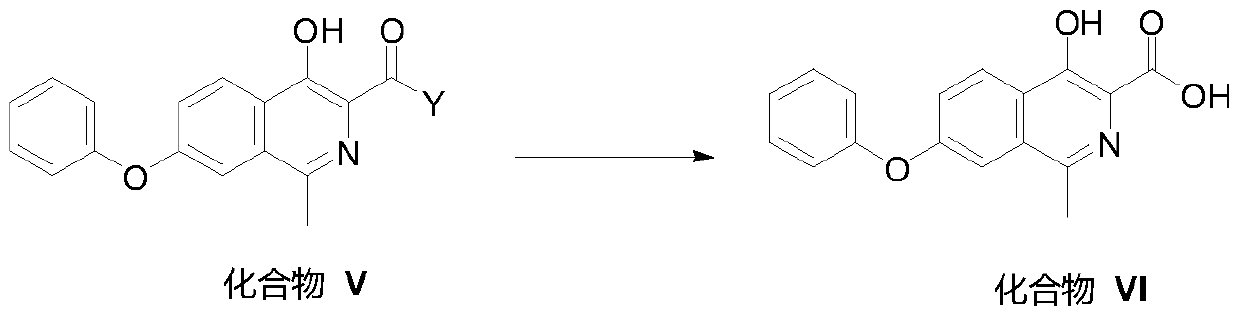
[0169] Embodiment 2 compound II-1 generates compound III-1
[0170]
[0171] Into a 250 mL three-necked flask, add 80 mL of dichloromethane, 15.62 g of compound II-1, 12.48 g of PyBOP, 2.92 g of tert-butylamine and 7.75 g of N,N-diisopropylethylamine, stir until incompletely dissolved, and leave overnight at room temperature. Suction filtration, add 80mL of water to the filtrate, separate layers, wash the organic layer with 1N hydrochloric acid 2×40mL, wash with saturated sodium bicarbonate 2×40mL, wash with pure water 40mL, and spin dry to obtain a white solid. Add 56mL of methanol for beating, filter with suction, and vacuum-dry the filter cake to obtain 5.1g of light yellow solid, yield: 75.8%. MS(+):m / z337.38(M+1); NMR data ( 1 H NMR, CDCl 3 ,400MHz): δppm 13.593(1H,s,OH),8.423(1H,s,CH),8.355-8.377(1H,d,J=8.8Hz,CH),8.004(1H,s,NH),7.436- 7.508(3H,q,CH),7.237-7.285(2H,q,CH),7.139-7.158(2H,d,J=7.6Hz,CH),1.556(9H,s,-C(CH) 3 ).
Embodiment 3
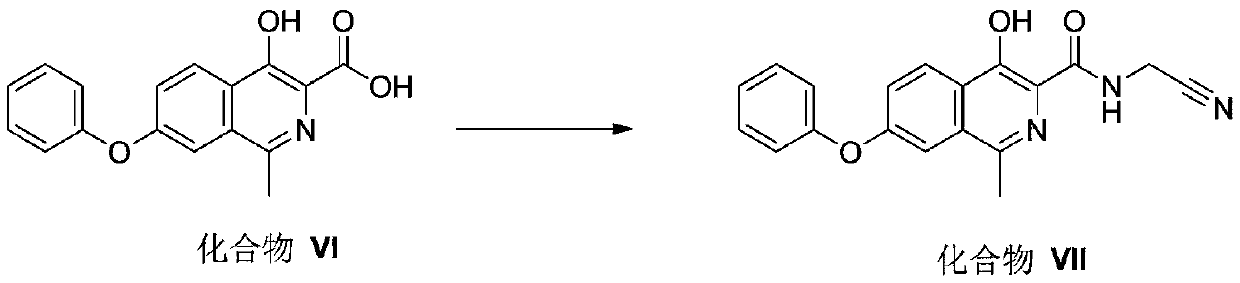
[0172] Embodiment 3 compound III-1 generates compound IV-1
[0173]
[0174] Into a 100mL three-neck flask, add 35mL of dichloromethane and 13.36g of compound III-1, dissolve it, add 1.72g of 1,3-dibromo-5,5-dimethylhydantoin, stir and heat to reflux for 6h. Cool down to 0-10°C, filter with suction, spin dry the filtrate to obtain 3.5 g of light red solid, yield: 84.3%. MS(+):m / z 416.28(M+1); NMR data ( 1 H NMR, CDCl 3 ,400MHz): δppm13.593(1H,s,OH),8.353-8.376(1H,d,J=9.2Hz,CH),7.667(1H,s,NH),7.647-7.653(1H,d,J= 2.4Hz, CH), 7.449-7.513 (3H, q, CH), 7.287 (1H, s, CH), 7.143-7.167 (2H, q, CH), 1.549 (9H, s, -C (CH) 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com