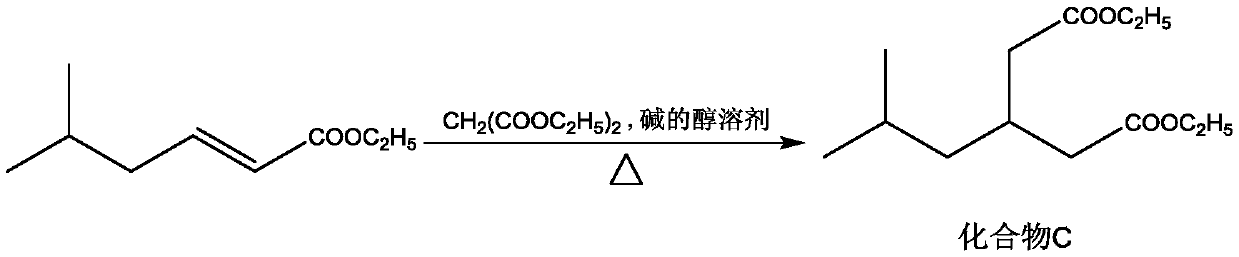
Method for preparing 3-isobutylglutaric acid
A technology of isobutylglutaric acid and isovaleraldehyde, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as low experimental yield, large steric hindrance, and long time consumption , to achieve the effect of ensuring the total yield, small steric hindrance, and simple reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Adopt the inventive method to prepare 3-isobutyl diethyl glutarate, comprise the steps:
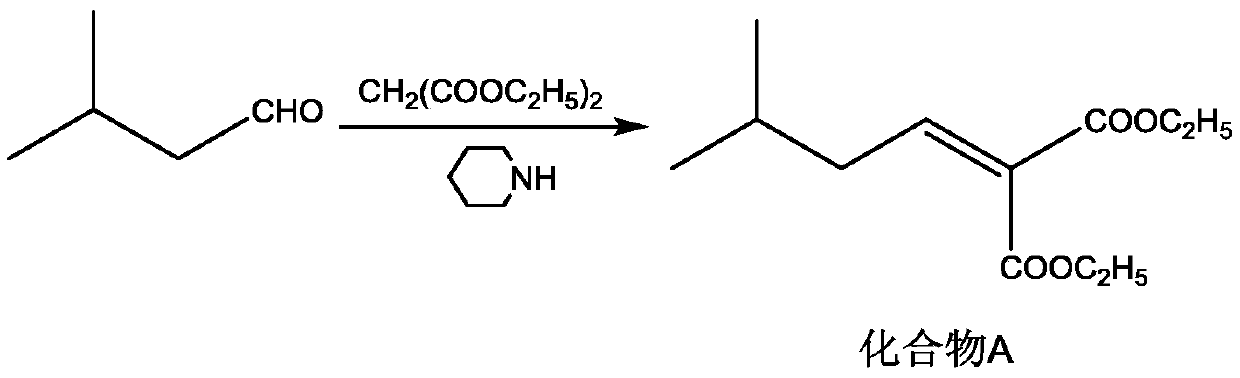
[0027] S1: Add diethyl malonate (16g, 0.1mol), isovaleraldehyde (8.6g, 0.1mol), cyclohexane (300mL), glacial acetic acid (30mL), hexahydropyridine (1mL ), install a water separator, and heat to reflux for 3h. Cool the reaction solution to room temperature, add saturated NaCl solution and stir for 0.5 h, let stand to separate layers, take the organic layer, and distill off most of the cyclohexane under reduced pressure to obtain a brown oil (compound A solution);
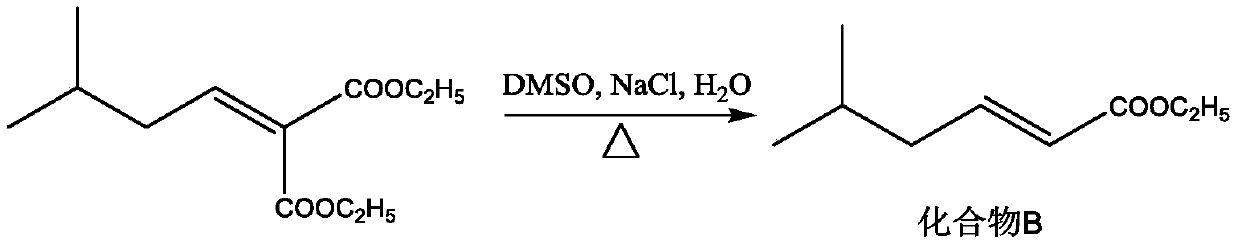
[0028] S2: Add the compound A solution, NaCl (11.3g, 0.194mol), DMSO (200mL) and water (5mL) in step S1 into the reaction flask, heat to 185°C under nitrogen protection, and stir for 3h. The reaction solution was cooled to room temperature, acidified by adding dilute hydrochloric acid, extracted with dichloromethane (200 mL / time x 3 times), washed with water, anhydrous Na 2 SO 4 After drying, most of the dichloromethan...
Embodiment 2
[0035] Adopt the inventive method to prepare 3-isobutyl diethyl glutarate, comprise the steps:
[0036] S1: Add diethyl malonate (20.8g, 0.13mol), isovaleraldehyde (10.3g, 0.12mol), cyclohexane (300mL), glacial acetic acid (30mL), hexahydropyridine ( 1mL), install a water trap, and heat to reflux for 3h. Cool the reaction solution to room temperature, add saturated NaCl solution and stir for 0.5 h, let stand to separate layers, take the organic layer, and distill off most of the cyclohexane under reduced pressure to obtain a brown oil (compound A solution);
[0037]S2: Add the compound A solution, NaCl (11.3g, 0.194mol), DMSO (200mL) and water (5mL) in step S1 into the reaction flask, heat to 185°C under nitrogen protection, and stir for 3h. The reaction solution was cooled to room temperature, acidified by adding dilute hydrochloric acid, extracted with dichloromethane (200 mL / time x 3 times), washed with water, anhydrous Na 2 SO 4 After drying, most of the dichloromethane...
Embodiment 3
[0044] Adopt the inventive method to prepare 3-isobutyl diethyl glutarate, comprise the steps:
[0045] S1: Add diethyl malonate (16g, 0.1mol), isovaleraldehyde (8.6g, 0.1mol), cyclohexane (300mL), glacial acetic acid (30mL), hexahydropyridine (1mL ), install a water separator, and heat to reflux for 3h. Cool the reaction solution to room temperature, add saturated NaCl solution and stir for 0.5 h, let stand to separate layers, take the organic layer, and distill off most of the cyclohexane under reduced pressure to obtain a brown oil (compound A solution);
[0046] S2: Add the compound A solution, NaCl (11.3g, 0.194mol), DMSO (200mL) and water (5mL) in step S1 into the reaction flask, heat to 185°C under nitrogen protection, and stir for 3h. The reaction solution was cooled to room temperature, acidified by adding dilute hydrochloric acid, extracted with dichloromethane (200 mL / time x 3 times), washed with water, anhydrous Na 2 SO 4 After drying, most of the dichloromethan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com