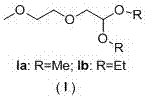
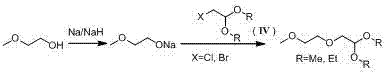
A kind of preparation method of 2-(2-methoxyethoxy) acetaldehyde acetal
A technology of methoxyethoxy and acetaldehyde acetal, which is applied in the field of preparation of 2-acetaldehyde acetal, can solve the problems of unfavorable large-scale industrialization, high risk of operation, and low product purity, and achieve easy Large-scale industrialization, easy purification, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of 2-(2-methoxyethoxy) acetaldehyde dimethyl acetal (I)
[0043]Add 91.2 g of ethylene glycol monomethyl ether into the reaction flask, and add 40 g of sodium hydroxide under stirring to obtain a mixture; Water drops evaporate again, stop heating, and lower the temperature to below 60°C; add 124.5 g of 2-chloroacetaldehyde dimethyl acetal, and then heat to 130°C for reflux condensation reaction for 8 hours until the solution no longer refluxes; after the reaction is completed, Cool to room temperature, filter, collect the filtrate, add 18.7g of sodium chloride, heat to 50-60°C and keep stirring for 7 hours, then distill under reduced pressure, vacuum degree -0.09 MPa, collect 110-116°C fractions to obtain 2- (2-Methoxyethoxy)acetaldehyde dimethyl acetal, 152.3 g, purity 98.5%, yield 92.8%.
Embodiment 2
[0045] The preparation method of 2-(2-methoxyethoxy) acetaldehyde diethyl acetal (I)
[0046] Add 228 g of ethylene glycol monomethyl ether into the reaction flask, add 40 g of sodium hydroxide under stirring to obtain a mixture, heat the mixture to 100-130 °C for dehydration by distillation under reduced pressure, vacuum degree -0.08 MPa; react until there are no more water drops Evaporate, stop heating, and lower the temperature to below 60°C; add 197.1 g of 2-bromoacetaldehyde diethyl acetal, then heat to 130°C and react for 6 hours, then cool to room temperature; filter, collect the filtrate, add 9.9 g of magnesium sulfate, Keep warm at 20-30°C and stir for 10 hours, then distill under reduced pressure, vacuum degree -0.10 MPa, collect fractions at 115-120°C to obtain 2-(2-methoxyethoxy)acetaldehyde diethyl acetal, 175.9 g , 98.2% purity, 91.6% yield.
Embodiment 3
[0048] The preparation method of 2-(2-methoxyethoxy) acetaldehyde dimethyl acetal (I)
[0049] Add 114 g of ethylene glycol monomethyl ether into the reaction flask, and add 40 g of sodium hydroxide under stirring to obtain a mixture; Water drops evaporate again, stop heating, and lower the temperature to below 60 °C; add 167 g of 2-bromoacetaldehyde dimethyl acetal, and then heat to 130 °C for reflux condensation reaction for 8 hours until the solution no longer refluxes; after the reaction is completed, Cool to room temperature, filter, collect the filtrate, add 16.7g of sodium sulfate, heat to 70-80°C and keep stirring for 2 hours, then distill under reduced pressure, vacuum degree -0.09 MPa, collect 110-116°C fractions to obtain 2-( 2-methoxyethoxy) acetaldehyde dimethyl acetal, 147.6 g, purity 98.5%, yield 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com