Emtricitabine and tenofovir medicine composition
A technology of emtricitabine and tenofovir fumarate, applied in the field of medicine, can solve the problems of easy degradation, high viscosity, affecting drug dissolution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
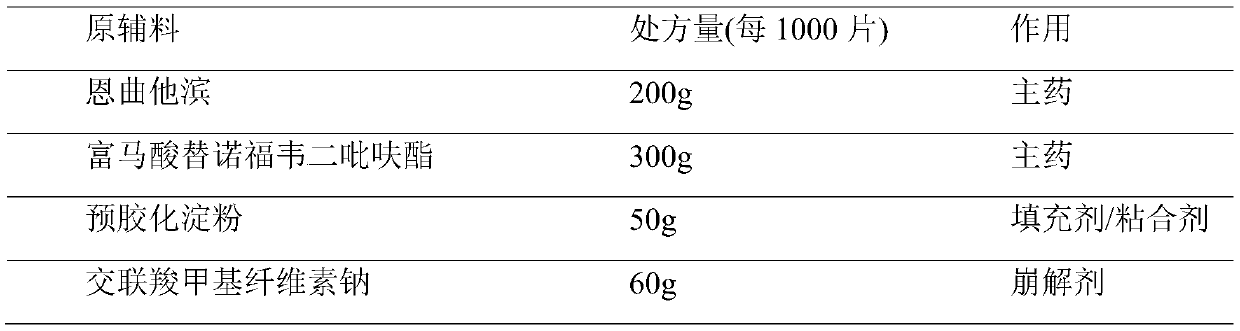
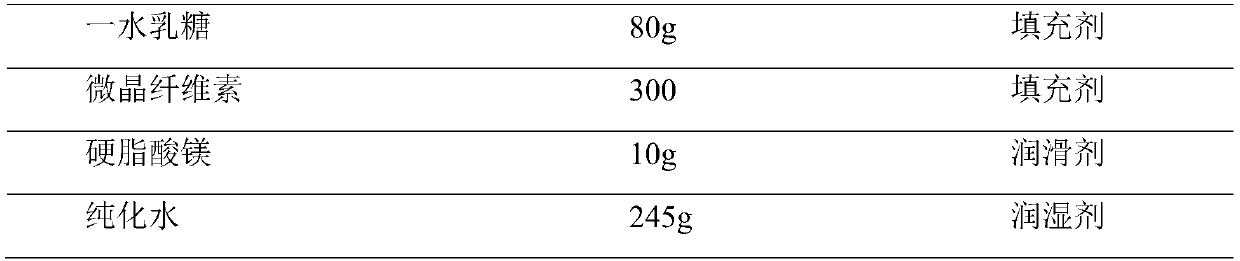
[0030]
[0031] Preparation method: Isopropanol is used as a granulation wetting agent, removed during the drying process, and floats within the range of 80% to 120% of the theoretical value according to specific conditions during the soft material production process. Special requirements for production environment: The following production environments require relative humidity below 50%. Disperse tenofovir disoproxil fumarate through a 40-mesh sieve for later use; crush emtricitabine and pass through an 80-mesh sieve for later use; dry mannitol at 85±5°C until the water content is less than 0.5 %, dry pregelatinized starch and croscarmellose sodium at 90±5°C until the moisture is less than 1.5%; dry magnesium stearate at 70±5°C until the moisture is less than 2.5%, and set aside; Starch, croscarmellose sodium, emtricitabine, tenofovir disoproxil fumarate, and mannitol were put into a multi-directional motion mixer and mixed to obtain a premixed medicinal powder. Put the ...
Embodiment 2
[0032] Example 2 (prepared with reference to comparative document CN1738628A)
[0033]
[0034]
[0035] Preparation method: Purified water is used as a granulation wetting agent, removed during the drying process, and floats within the range of 80% to 120% of the theoretical value according to specific conditions during the soft material production process. Special requirements for production environment: The following production environments require relative humidity below 50%. Disperse tenofovir disoproxil fumarate through a 40-mesh sieve for subsequent use; pulverize emtricitabine and pass through an 80-mesh sieve for subsequent use; mix lactose monohydrate, pregelatinized starch, microcrystalline cellulose and Sodium carboxymethyl cellulose is dried at 90±5°C until the water content is less than 1.5%; magnesium stearate is dried at 70±5°C until the water content is less than 1.5%, and it is ready for use; croscarmellose sodium, emtricitabine, Put tenofovir disoprox...
Embodiment 3
[0037] Get the sample prepared according to Examples 1-2 and the original research product, according to the dissolution assay method (Chinese Pharmacopoeia 2015 edition four general rules 0931 second method), the dissolution comparative results in the hydrochloric acid solution of 900ml 0.01mol / L are shown in Table 1 ~2.
[0038] Emtricitabine dissolution curve result in the present invention and commercially available product Truvada in table 1
[0039]
[0040] Table 2 Dissolution curve results of tenofovir disoproxil in the present invention and commercially available product Truvada
[0041]
[0042]
[0043] The results show that the present invention releases faster than the original research product, and has a faster dissolution advantage as an immediate release preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com