Preparation method of melanin particles
A technology of melanin particles and melanin precursors, which is applied in the field of melanin synthesis, can solve the problems of uneven particle size and irregular spherical structure, and achieves the effects of uniform particle size, good dispersion effect and good ultraviolet absorption effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Weigh 0.18g levodopa (Shanghai Aladdin Biochemical Technology Co., Ltd., specification: AR) and dissolve it in 90ml deionized water (the concentration of the aqueous solution containing levodopa is 2mg / ml at this time), add 1mol / L NaOH solution ( Sinopharm Group Chemical Reagent Co., Ltd., specification: AR) is adjusted to system pH and is 7.2, adds 0.06g potassium ferrate (Shanghai McLean Biochemical Technology Co., Ltd., specification: AR), and now the mol ratio of levodopa and oxidant is 1: 0.33, 90°C, after stirring for 6 hours, the reaction ends, slowly add 1mol / L hydrochloric acid solution dropwise to make the system pH≤7. After centrifugal drying, melanin 1 was obtained, with an average diameter of about 78nm.
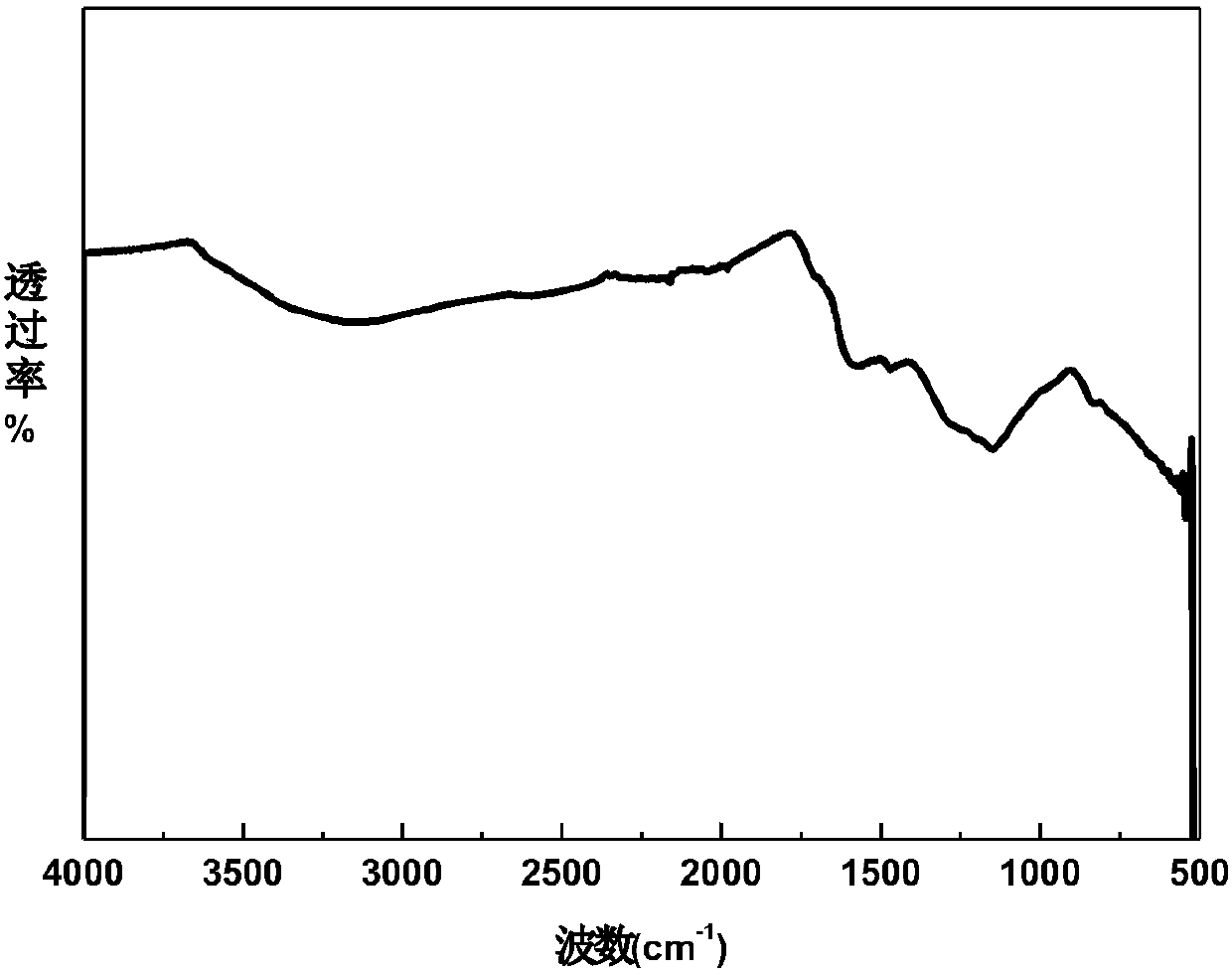
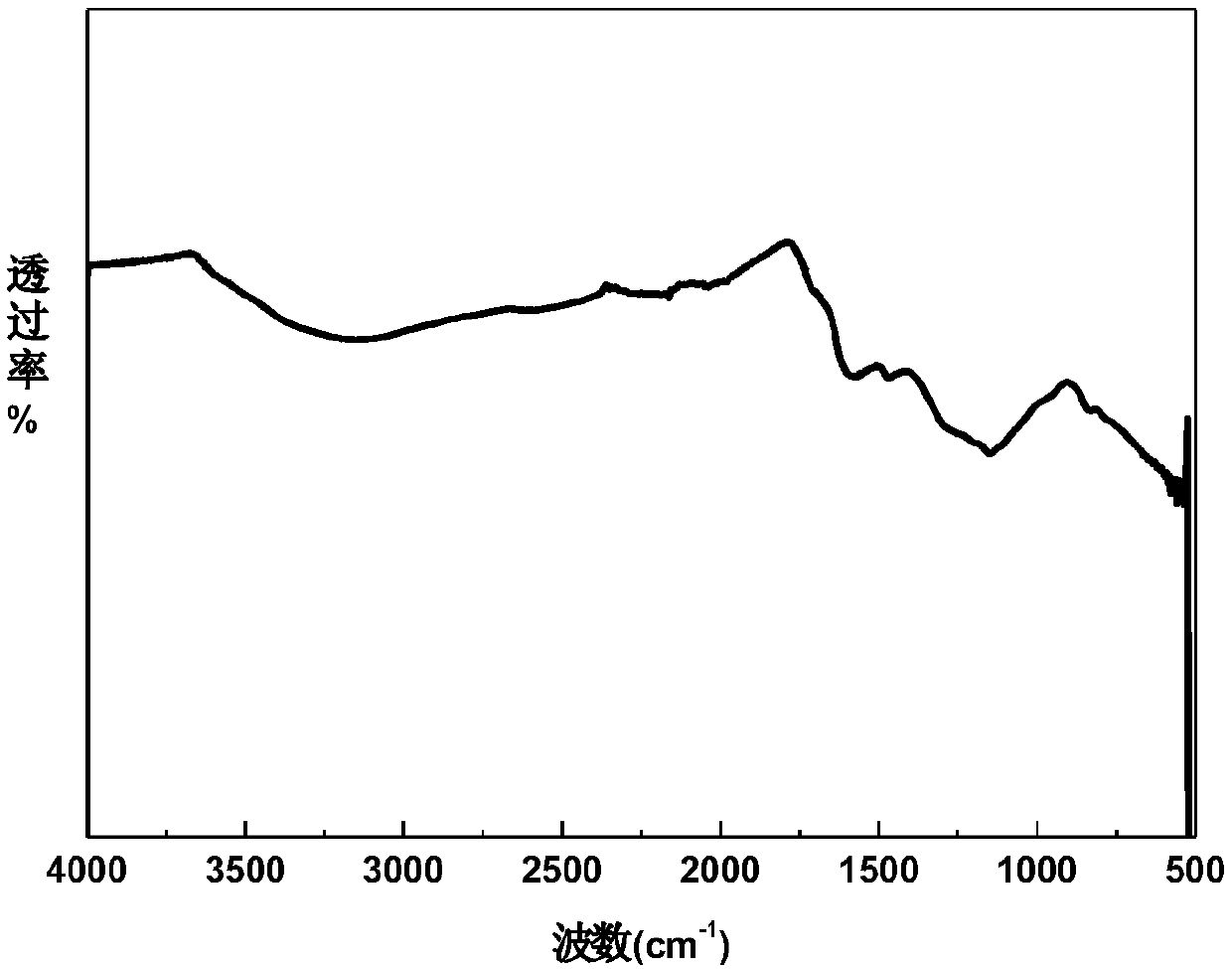
[0040] combine Figure 2 to Figure 5 It can be seen that both melanin 1 and natural melanin have almost the same infrared characteristic peaks, specifically at 3500-3200cm -1 Occurrence of -OH, -NH 2 And the N-H stretching vibration of the indole struc...
Embodiment 2
[0043]Weigh 0.18g levodopa (Shanghai Aladdin Biochemical Technology Co., Ltd., specification: AR) and dissolve it in 90ml deionized water (the concentration of the aqueous solution containing levodopa is 2mg / ml at this time), add 1mol / L NaOH solution ( Sinopharm Group Chemical Reagent Co., Ltd., specification: AR) is adjusted to system pH and is 8.6, adds 0.06g potassium ferrate (Shanghai McLean Biochemical Technology Co., Ltd., specification: AR), and now the mol ratio of levodopa and oxidant is 1: 0.33, 90°C, after stirring for 5 hours, the reaction ends, slowly add 1mol / L hydrochloric acid solution dropwise to make the system pH≤7. After centrifugal drying, melanin 2 was obtained, with an average diameter of about 50 nm.
[0044] Weigh 5g polycarbonate (DOW Chemical Company, USA, weight average molecular weight is about 30000) in 30g cyclohexanone (Sinopharm Chemical Reagent Co., Ltd., specification: AR), heat and stir to dissolve it completely. Take 0.0025g of melanin 2 p...
Embodiment 3
[0046] Weigh 0.18g of L-tyrosine (Shanghai Aladdin Biochemical Technology Co., Ltd., specification: AR) and dissolve it in 180ml of deionized water (at this time, the concentration of the aqueous solution containing L-tyrosine is 1mg / ml), add 1mol / LNaOH solution (Sinopharm Chemical Reagent Co., Ltd., specification: AR) was adjusted to a system pH value of 8.6, and 0.018 g of potassium ferrate (Shanghai Macklin Biochemical Technology Co., Ltd., specification: AR) was added. At this time, L-tyrosine The molar ratio to the oxidizing agent is 1:0.1, at 90°C, after stirring for 5 hours, the reaction ends, and slowly add 1 mol / L hydrochloric acid solution dropwise to make the system pH≤7. After centrifugal drying, melanin 3 was obtained, with an average diameter of about 30 nm.
[0047] Weigh 5g polycarbonate (DOW Chemical Company, USA, weight average molecular weight is about 30000) in 30g cyclohexanone (Sinopharm Chemical Reagent Co., Ltd., specification: AR), heat and stir to di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com