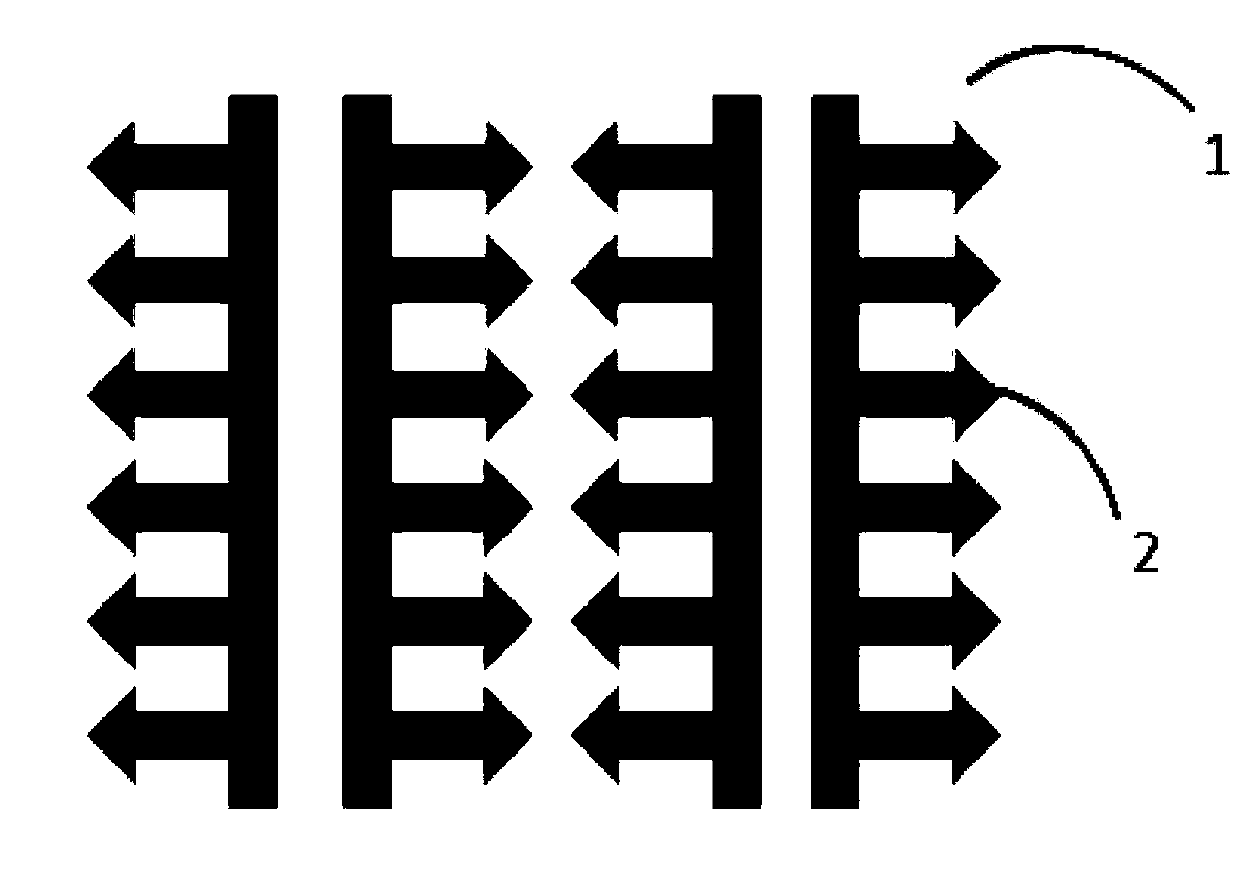
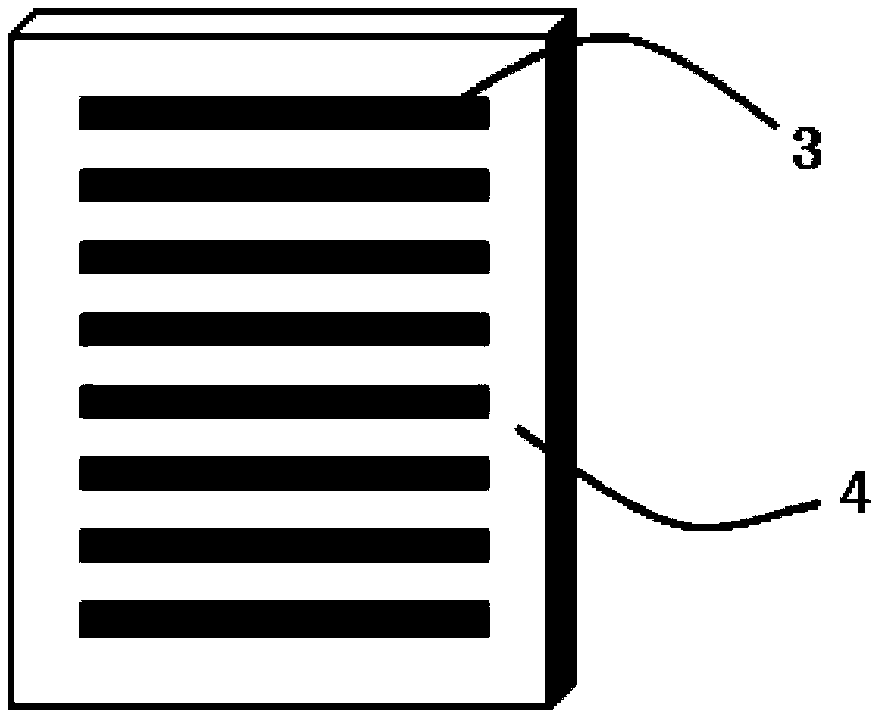
Preparation method of soluble microneedle patch for rabies vaccine
A rabies vaccine and micro-needle sticker technology, applied in the directions of micro-needles, needles, and medical devices, can solve the problems of easy breakage of the needle body, increased manufacturing costs, residues, etc., and achieves flexible assembly methods, excellent mechanical properties, and flexible shapes. control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 rabies vaccine soluble microneedle patch
[0039] (1) Preparation of PDMS transverse mold by UV-LIGA method
[0040] First, mix PDMS and curing agent evenly at a mass ratio of 10:1, place in a vacuum drying oven, and evacuate for 5 minutes at a vacuum degree of 0.08Mpa to remove air bubbles in the solution, and let it stand for later use;
[0041] Secondly, prepare a silicon substrate, sputter a layer of nickel on the silicon substrate, and pre-treat it to ensure absolute cleanliness;
[0042] Then, throw a layer of 100 μm thick SU-8 glue on the pretreated silicon substrate, and use a mask for photolithography development. The shape of the mask is the same as that of the needle body of the microneedle;
[0043] Finally, pour the aforementioned vacuum-pumped PDMS solution, place it in an oven, dry it at 60°C for 5 hours, take it out, and demould it to obtain a PDMS transverse mold.
[0044] The shape of the needle body in the present inv...
Embodiment 2
[0056] The needle type comparison of the microneedle that embodiment 2 different preparation methods make
[0057] Prepare soluble microneedles respectively according to the method of Example 1 of the present invention and the traditional mold-inversion method, place the prepared soluble microneedles under a scanning electron microscope to measure and calculate the average height of the microneedles after demoulding, and obtain the height uniformity The percentage of microneedles under the premise (no significant difference, P greater than 0.05), the height of both molds is 500 μm, the number of needles is 50, and 20 pieces are parallel. The calculation results are shown in Table 1.
[0058] Table 1 Investigation of the height uniformity of microneedles prepared by different preparation methods
[0059] Preparation traditional casting method The method of the invention Average height of microneedles (μm) 446.5* 486.7* Highly uniform acquisition rate ...
Embodiment 3
[0062] Example 3 Comparison of Microneedles Prepared by Different Preparation Methods in Skin Piercing and Peeling Experiments
[0063] Prepare the rabies vaccine soluble microneedle patches with the same number of needles and the same backing area according to the mold-inversion method, 3D printing method, and the method of Example 1 of the present invention, and use an applicator to puncture the soluble microneedle patches prepared by the three methods. Into the isolated pig skin, to ensure that the three types of microneedles have pierced the skin. After 1 min of administration, the backing layer of the microneedles was peeled off, and the conditions of the peeled off microneedles and the backing layer were observed.
[0064] The observation results are shown in Table 2. As can be seen from Table 2, only adopt the method needle body of the present invention to all stay in the skin, show that rabies vaccine can be fully inoculated.
[0065] Table 2 Summary table of the sit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com