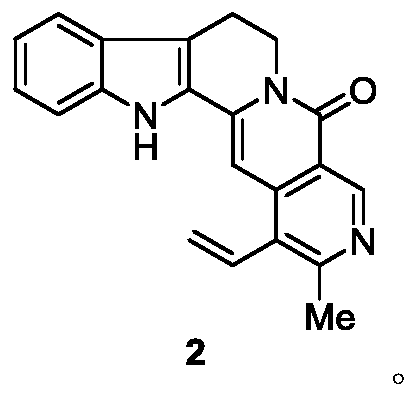
Preparation method and application of 21-methyl-subditine
A technology of strychnine and methyl, which is applied in the field of chemical drug synthesis, can solve the problems of difficult chemical synthesis and chemical synthesis without subditine, and achieve the effect of strong practical value, strong superiority, and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
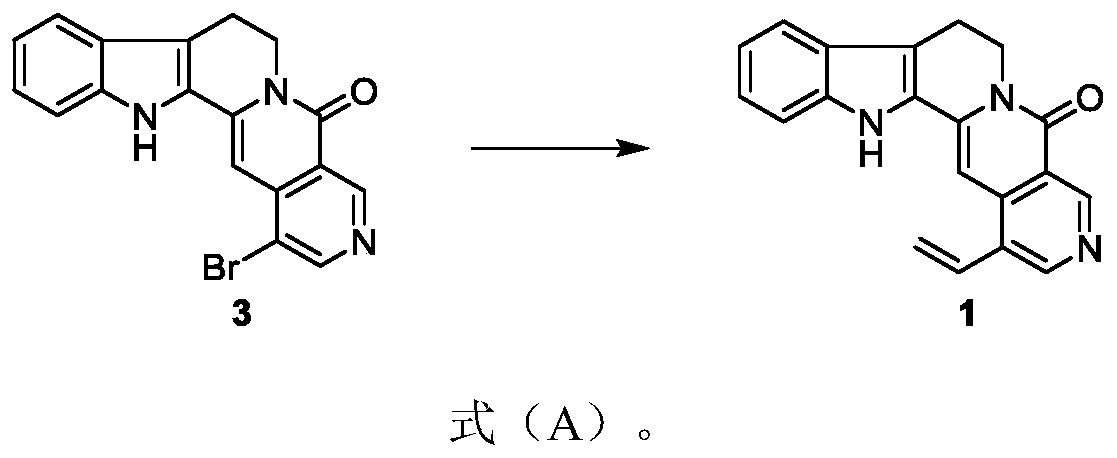
[0059] Embodiment 1: the preparation of formula 1 compound
[0060]
[0061] Put the compound of formula 3 (15.2mg, 0.042mmol) and bis(triphenylphosphine) palladium dichloride (4.8mg, 0.042mmol) in a branched reaction flask, and after three gas changes, the system was placed in a nitrogen atmosphere , add a 1:1 mixed solution of toluene and N,N-dimethylformamide (1.50ml) and tributylvinyltin (13ul, 0.046mmol) to the system successively, and put it at 100°C for 3 hours to react. Completely, cool the system to room temperature, after suction filtration, dilute with ethyl acetate solution, wash the organic phase with distilled water and saturated brine successively, dry the organic phase with anhydrous sodium sulfate, spin off the solvent, and wash the concentrate with 50% ethyl acetate / dichloromethane eluent carries out column chromatography and obtains solid product formula 1 (6.8g, 79%), R f = 0.28 (5% methanol / dichloromethane).
[0062] Tested: 1 H NMR (500MHz, DMSO-d ...
Embodiment 2
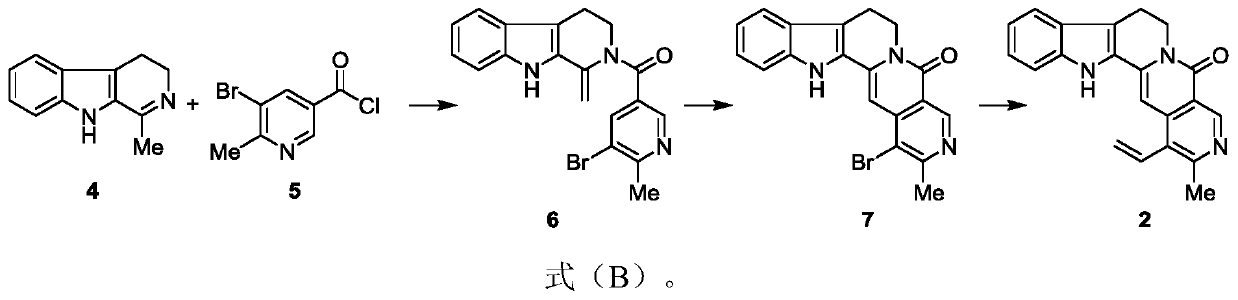
[0064] Embodiment 2: the preparation of formula 6 compound
[0065]
[0066] Dissolve the compound of formula 5 (500 mg, 2.15 mmol) in dichloromethane (15 ml) solution, add triethylamine (6 ml), stir at room temperature for 1 hour, and dissolve the compound of formula 4 (250 mg, 1.34 mmol) in the above system, Put it at 45°C to start the reaction, react for 2 hours, the reaction is complete, spin off the solvent, and the concentrate is separated by column chromatography with 25% ethyl acetate / petroleum ether eluent to obtain the compound of formula 6 (250mg, yield 49%) , R f =0.63 (30% ethyl acetate / petroleum ether).
Embodiment 3
[0067] Embodiment 3: the preparation of formula 7 compound
[0068]
[0069] Put the compound of formula 6 (250mg, 0.65mmol) in a round-bottomed flask, heat up to 190°C under vacuum, react for 30 minutes, the reaction is complete, cool to room temperature, concentrate with 50% ethyl acetate / dichloromethane The eluent was separated by column chromatography to obtain the compound of formula 7 (120mg, yield 48%), R f = 0.65 (10% methanol / dichloromethane).
[0070] 1 H NMR (500MHz, Chloroform-d) δ9.40(s, 1H), 7.65(d, J=7.9Hz, 1H), 7.49(d, J=8.2 Hz, 1H), 7.37(ddd, J=8.2, 7.0, 1.1Hz, 1H), 7.23(t, J=7.5Hz, 4H), 6.87(s, 1H), 4.54(t, J=6.7Hz, 2H), 3.20(t, J=6.7Hz, 2H) , 2.83(s, 3H).
[0071] 13 C NMR(126MHz,Chloroform-d)δ161.41,159.48,149.34,141.59,138.48,137.42, 127.23,125.92,125.53,120.99,119.88,119.12,117.19,116.79,111.78,96.15,40.64, 26.11,19.79ppm. HRMS (ESI, m / s): [M+H] + calcd.for C 19 h 15 N 3 OBr, 380.0398; found 380.0393.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com