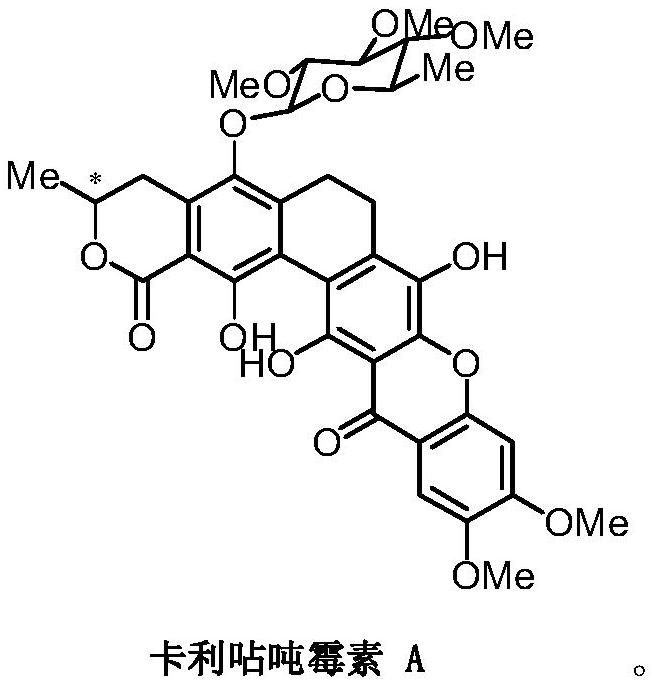
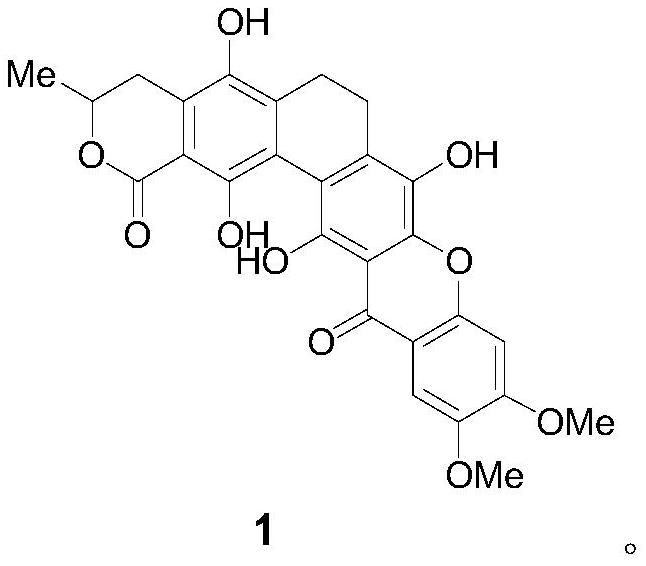
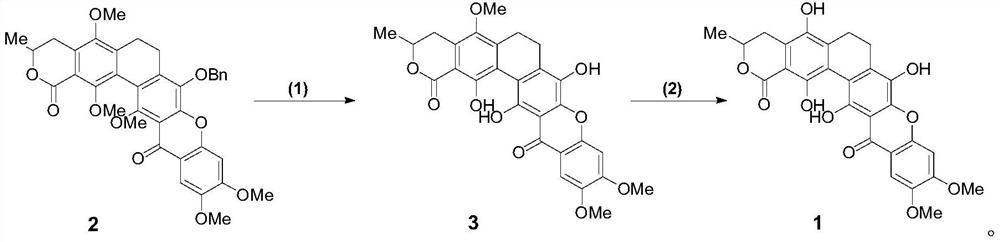
A glycosyl ligand of calixanthamycin A and its intermediate and preparation method
A xanthomycin and glycosyl technology, applied in the field of chemical drug synthesis, can solve the problem that there are no reports related to chemical synthesis of calixanthomycin A glycosyl ligands, the high oxidation state is complex, and there is no chemical synthesis of calixanthene. Mycin A, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] Embodiment 1: the preparation of formula 10 compound
[0131] Step a: Preparation of the compound of formula 10-2:
[0132]
[0133] Dissolve the compound of formula 10-1 (10.0g, 49.2mmol) in acetone (120ml), add potassium carbonate (10.2g, 73.9mmol), tetrabutylammonium fluoride (182mg, 0.49mmol), stir and mix well, add Allyl bromide (5.1ml, 59.1mmol) was then reacted at 55°C for 12 hours to end the reaction, the reaction solution was cooled to room temperature, filtered with diatomaceous earth, concentrated, and the residue was column chromatographed (5% ethyl acetate / petroleum ether) to obtain the compound of formula 10-2 (11.77 g, yield 98%), Rf=0.54 (10% ethyl acetate / petroleum ether).
[0134] Tested: 1 H NMR (500MHz, Chloroform-d) δ7.14(d, J=2.9Hz, 1H), 6.85(d, J=8.9Hz, 1H), 6.80(d, J=8.9Hz, 1H), 6.07(m ,1H),5.48(dq,J=17.2,1.6Hz,1H),5.31(d,J=10.6Hz,1H),4.55(d,J=3.7Hz,2H),3.76(s,3H);
[0135] 13 C NMR (125MHz, CDCl3) δ154.3, 149.3, 133.1, 118.9, 117.6, 115....
Embodiment 2
[0175] Embodiment 2: the preparation of formula 11 compound
[0176] Step A: Preparation of compound of formula 11-2:
[0177]
[0178] The formula 11-1 compound (7.20g, 30.9mmol, 1.0equiv.) was dissolved in 60ml of DMF, potassium carbonate (6.40g, 46.3mmol, 1.5equiv.) and benzyl bromide (4.0ml, 34.0mmol, 1.1equiv.) were added .), react at room temperature for 2 hours, finish the reaction, add 20ml of distilled water to quench the reaction, extract three times with ethyl acetate (30ml), combine the organic phases, wash the organic phases with distilled water for 3 times, and wash with saturated sodium chloride solution for 1 time , dried over anhydrous sodium sulfate, filtered, concentrated, and the residue was separated by column chromatography (5% ethyl acetate / petroleum ether) to obtain the compound of formula 11-2 (8.97g, yield 90%), Rf=0.57 ( 10% ethyl acetate / petroleum ether).
[0179] Tested: 1 H NMR (400MHz, CDCl3) δ10.04(s, 1H), 7.43(d, J=3.1Hz, 1H), 7.42–7.34(m...
Embodiment 3
[0203] Embodiment 3: the preparation of formula 7 compound
[0204]
[0205] Formula 10 compound (1.52g, 5.05mmol, 1.0equiv.) and formula 11 compound (2.70g, 6.56mmol, 1.3equiv.) are dissolved in the mixed solvent of the DMF of 40ml and the triethylamine of 8ml, then add Ph( PPh3) 4 (577mg, 0.5mmol, 0.1equiv.) and CuI (95.3mg, 0.5mmol, 0.1equiv.), after the addition, under the protection of nitrogen, reacted at 95°C for 10.5 hours, ended the reaction, and lowered the temperature of the reaction system to room temperature, with 10ml saturated NH 4 Cl aqueous solution quenched reaction, extracted three times with ethyl acetate (40ml), combined organic phase, organic phase was washed twice with distilled water and saturated saline, dried, filtered, and concentrated to obtain formula 7 compound (2.50g, yield 78%).
[0206] Tested: 1 H NMR (500MHz, Chloroform-d) δ7.58–7.49(m,2H),7.32(q,J=6.2Hz,3H),6.85(s,1H),6.66(d,J=3.0Hz,1H) ,6.57(d,J=2.9Hz,1H),5.14(s,2H),4.52(ddd,J=11.6,6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com