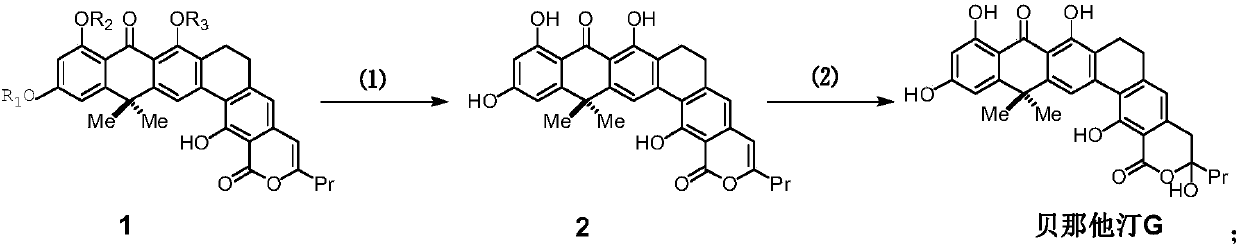
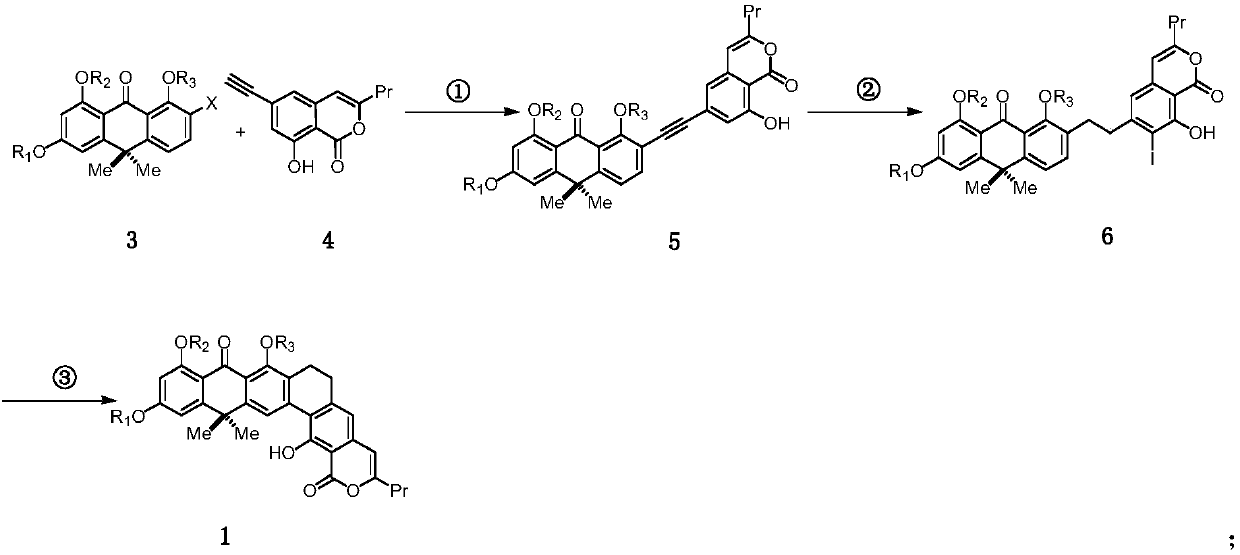
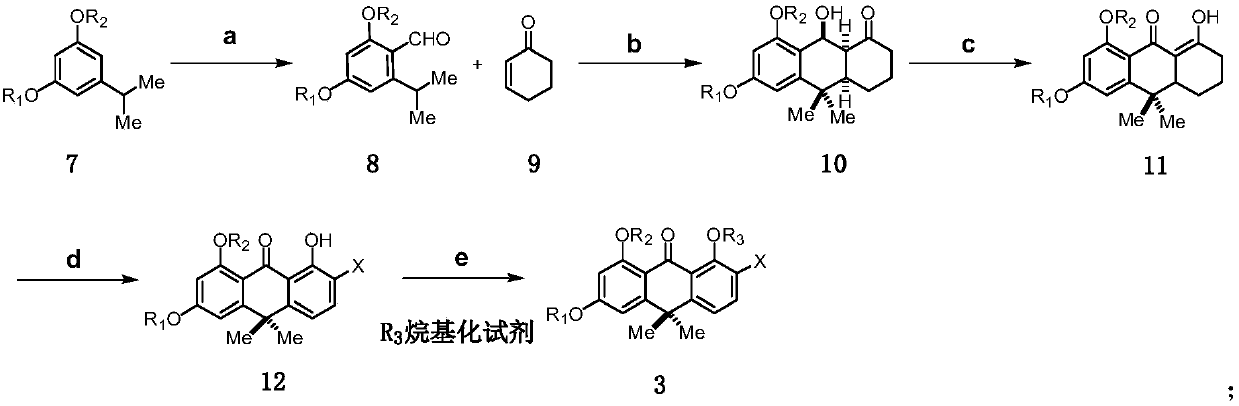
Method for synthesizing benastatin G
A technology of benastatin and synthetic route, applied in the field of medicinal chemical synthesis, can solve the problems of complex chemical structure, no chemical synthesis of benastatin G, difficulty in chemical synthesis of benastatin G, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Embodiment 1: Compound shown in preparation formula 3:
[0117] Step a: prepare the compound shown in formula 8:
[0118]
[0119] The compound of formula 7 (3.51g, 19.49mmol) was dissolved in N,N-dimethylformamide (20mL), and then phosphorus oxychloride (2.68mL, 29.23mmol) was slowly added dropwise at 0°C under nitrogen protection, After the dropwise addition, stir the reaction at 90°C for 3 hours to end the reaction. After the reaction solution is cooled to room temperature, it is slowly poured into ice water. The resulting mixed solution is adjusted to pH 10 with 20 wt% sodium hydroxide solution, and then extracted with ether. , the organic phases were combined, and the organic phase obtained was washed once with saturated sodium bicarbonate solution and saturated brine successively, dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure until no solution was distilled off, and the residue was analyzed by column chr...
Embodiment 2
[0147] Embodiment 2 prepares the compound shown in formula 4:
[0148] Step A: prepare the compound shown in formula 14:
[0149]
[0150] The compound of formula 13 (8.08g, 25.58mmol) was dissolved in N,N-dimethylformamide (64mL), and potassium carbonate (7.78g, 56.27mmol) and dimethyl sulfate (5.09mL, 53.72mmol) were added, Then react at 60°C for 21.5 hours to end the reaction, cool the reaction liquid to room temperature, quench with saturated ammonium chloride solution, extract with ethyl acetate, combine the organic phases, and wash the organic phases twice with water, saturated chlorinated Sodium was washed once, dried with anhydrous sodium sulfate, filtered, concentrated, and the residue was separated by column chromatography (5% ethyl acetate / petroleum ether) to obtain the compound of formula 14 (7.74g, yield rate 88%), Rf= 0.56 (20% ethyl acetate / petroleum ether).
[0151] Tested: 1 H NMR (300MHz, CDCl 3 )δ6.46(s,2H),3.91(s,3H),3.83(s,6H)ppm;
[0152] 13 C NM...
Embodiment 3
[0196] Embodiment 3 prepares the compound shown in formula 1:
[0197] Step ①: prepare the compound shown in formula 5:
[0198]
[0199] The compound of formula 3 (907mg, 2.07mmol) was dissolved in a mixed solvent of N,N-dimethylformamide (5mL) and diisopropylamine (3mL), and tetrakistriphenylphosphine palladium (239.2mg, 0.207mmol) was added , cuprous iodide (19.7mg, 0.104mmol), after freezing and deoxygenating with liquid nitrogen, add 2.5mL of N, N-dimethylformamide solution of formula 4 compound (496.2mg, 2.17mmol) of freezing and deoxygenating, and then React at 45°C for 3 hours to end the reaction. After cooling the reaction liquid to room temperature, add saturated ammonium chloride to quench, extract with ethyl acetate, combine the organic phases, wash the obtained organic phases twice with distilled water, and then with saturated sodium chloride. Once, dried over anhydrous sodium sulfate, filtered, concentrated, and the residue was separated by column chromatogra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com