Method for synthesizing thiazolecarboxylic acid chemical intermediate key intermediate product
A synthesis method and technology of thiazole carboxylic acid, applied in the direction of organic chemistry, etc., can solve the problems of poor product yield and purity, difficult to meet the needs of biomedical synthesis, outdated, etc., and achieve high yield and purity, which is beneficial to industrialized mass production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
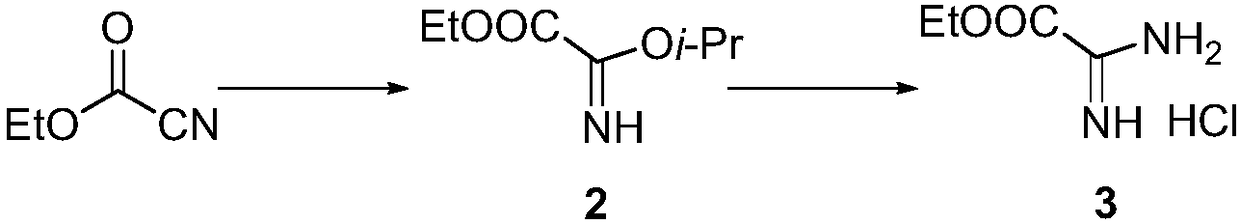
[0012] The synthetic method of key intermediate product of thiazole carboxylic acid chemical intermediate of the present invention, such as figure 1 As shown, it includes: (a) Mix ethyl cyanoformate, isopropyl ether and isopropanol, lower the temperature to -20°C to -5°C under the protection of an inert gas, and feed HCl gas under stirring conditions Carry out the reaction, let it stand to room temperature, and suction filter to obtain a white solid intermediate; (b) stir and mix water, sodium bicarbonate, ice and methyl tert-butyl ether, add the intermediate, and keep the water temperature below 0 ℃ and the pH is weakly alkaline to free the intermediate; separate the organic phase, dry and filter, and concentrate the filtrate under reduced pressure to obtain a transparent oily compound 2; (c) add the compound 2 to ethanol, under the protection of an inert gas Lower the temperature with an ice bath, add ammonium chloride to react; concentrate under reduced pressure to remove e...
Embodiment 1
[0015] This embodiment provides a kind of synthetic method of key intermediate product of thiazole carboxylic acid chemical intermediate, and it comprises:
[0016] (a) Add ethyl cyanoformate (100g, 1.0mol, 1.0eq), isopropyl ether (800ml) and isopropanol (67g, 1.1mmol, 1.1eq) in a 2L three-necked flask, 2 The temperature was lowered to -10°C under protection, and HCl gas was introduced to react for 15 hours under stirring, and allowed to stand at room temperature, and filtered with suction to obtain a white solid intermediate;
[0017] (b) Add 1L of water, 250g of sodium bicarbonate, 200g of ice, and 350mL of methyl tert-butyl ether into a 2L reaction flask. After stirring and mixing, add the above-mentioned intermediate while keeping the water temperature below 0°C and the pH at weakly alkaline (pH value is 8~9), dissociation completes (is about to disperse the intermediate in the above-mentioned mixture), separates the organic phase, 100L is extracted with methyl tert-butyl ...
Embodiment 2
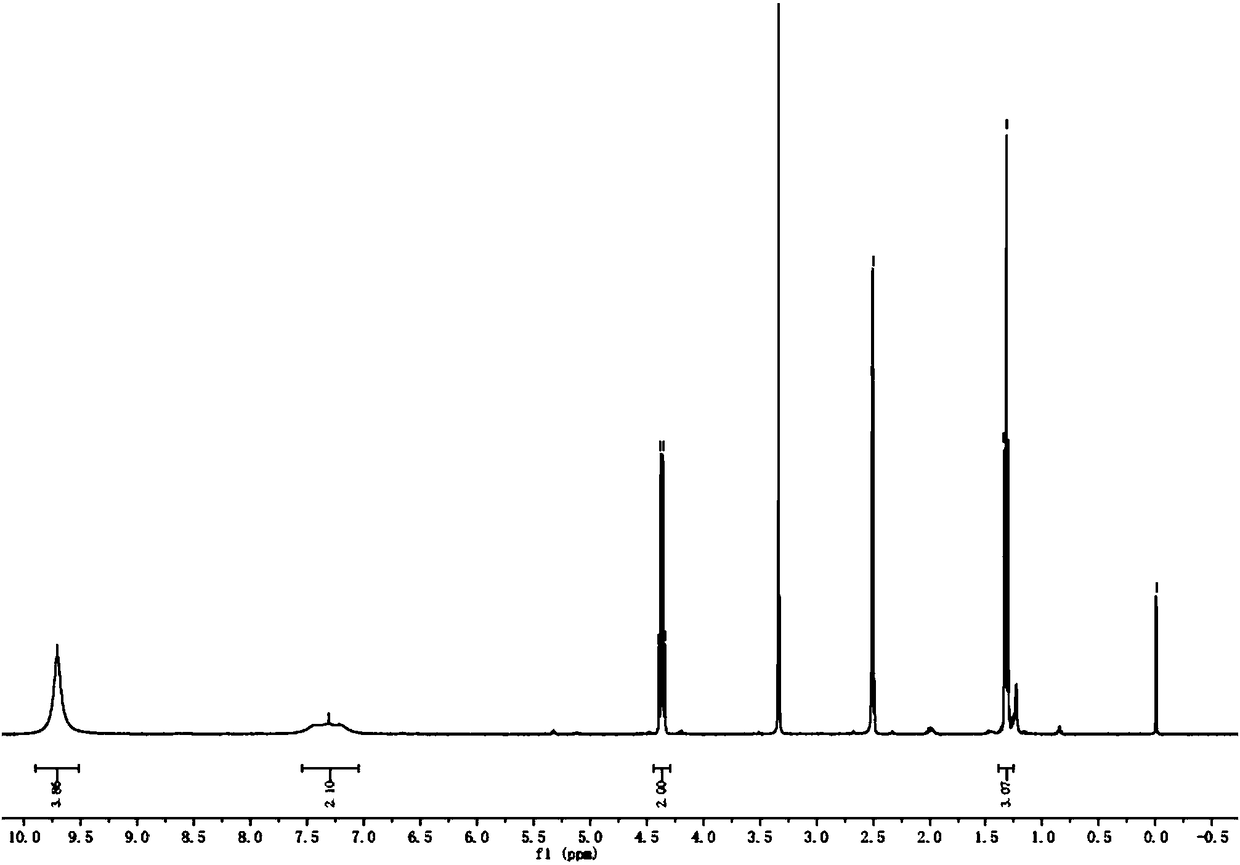
[0020] This embodiment provides a synthetic method for key intermediates of thiazole carboxylic acid chemical intermediates, which is basically the same as in Example 1, except that in step (b), the organic phase is separated without methyl tert-butyl Extracted with base ether to obtain 104g of transparent oily liquid compound 2 (the yield of step a and step b is 68%, and the purity is 98.0%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com