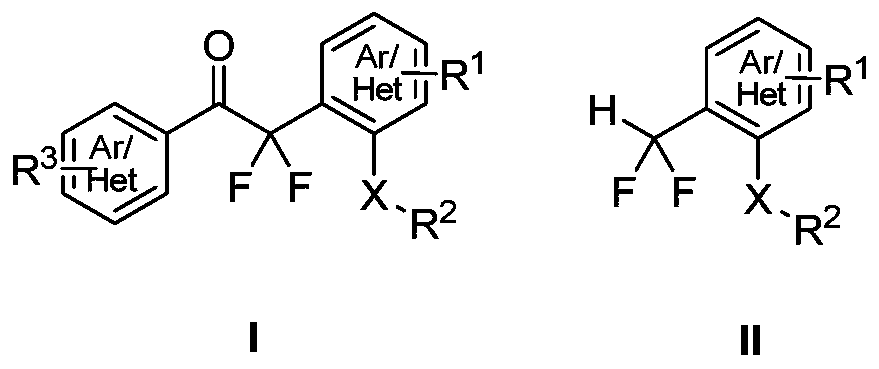
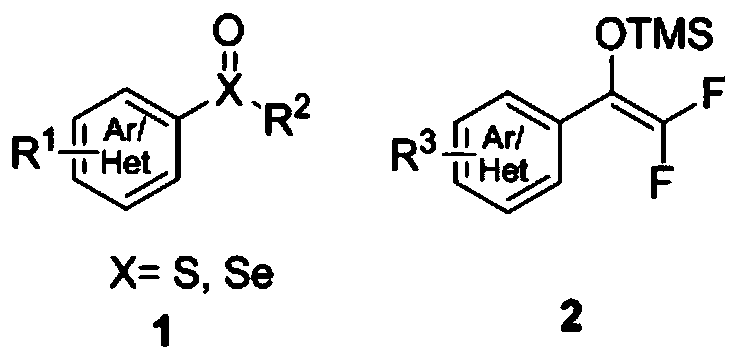
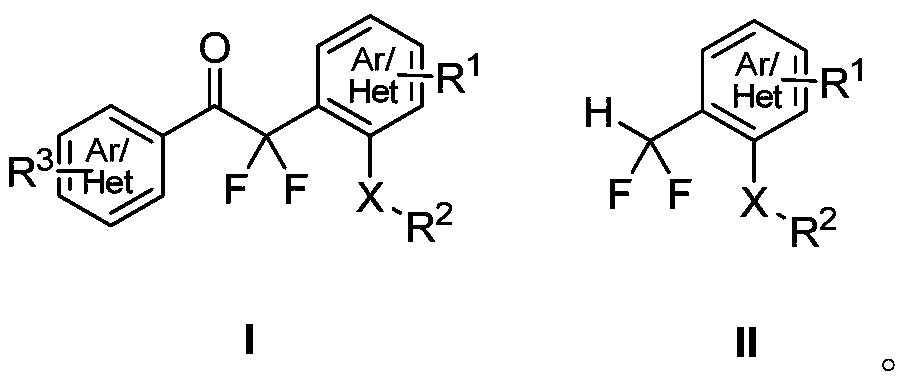
Method for synthesizing difluoroalkyl or difluoromethyl sulfur-containing or selenium-containing compounds
A technology of difluoromethyl and difluoroalkyl, applied in the preparation of sulfide, organic chemistry, etc., can solve the problem of no difluoroalkyl or difluoromethyl, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In a 50 mL dry reaction tube, add diphenyl sulfoxide (202.3 mg, 1.0 mmol), phenyl difluoroenol silyl ether (342.5 mg, 1.5 mmol), ethyl acetate 10.0 mL, and then dropwise add acetic anhydride ( 153.1mg, 1.5mmol), magnetically stirred at -40°C for 24 hours, detected by TLC, the raw material disappeared, concentrated directly, and further purified by column chromatography to obtain the product with a yield of 99%;
[0052] 2,2-Difluoro-1-phenyl-2-(2-(phenylthio)phenyl)ethanone (3a)
[0053] 1 H NMR (400MHz, CDCl 3 )δ7.98(d, J=7.4Hz, 2H), 7.86(dd, J=7.6, 1.7Hz, 1H), 7.63–7.53(m, 1H), 7.48–7.36(m, 4H), 7.32(d ,J=7.4Hz,1H),7.25–7.17(m,3H),7.11–7.03(m,2H); 19 F NMR (376MHz, CDCl 3 )δ–93.63(s,2F); 13 C NMR (100MHz, CDCl 3 ): δ189.0(t, J=31.0Hz), 135.7, 135.6(t, J=23.0Hz), 134.6(t, J=4.0Hz), 134.5, 133.5, 133.3(t, J=2.1Hz), 131.3, 130.4, 129.8 (t, J = 2.6Hz), 129.0, 128.4, 127.6, 127.2, 126.5 (t, J = 8.6Hz), 116.4 (t, J = 254.4Hz); HRMS (ESI) calcd for [C 20 h 14 f 2 O...
Embodiment 2
[0055] In a 50mL dry reaction tube, add 4,4'-xylene sulfoxide (230.3mg, 1.0mmol), phenyl difluoroenol silyl ether (342.5mg, 1.5mmol), dichloromethane 10.0mL, then drop Add trifluoromethanesulfonic anhydride (423.2mg, 1.5mmol), stir magnetically at -30°C for 24 hours, detect by TLC, the raw material disappears, concentrate directly, and then further purify by column chromatography to obtain the product with a yield of 93%;
[0056] 2,2-Difluoro-2-(5-methyl-2-(p-tolylthio)phenyl)-1-acetophenone (3b)
[0057] 1 H NMR (400MHz, CDCl 3 )δ7.98(dd,J=8.3,0.9Hz,2H),7.67–7.65(m,1H),7.61–7.53(m,1H),7.46–7.38(m,2H),7.20(d,J= 1.1Hz, 2H), 7.01(d, J=8.0Hz, 2H), 6.99–6.91(m, 2H), 2.43(s, 3H), 2.29(s, 3H); 19 FNMR (376MHz, CDCl 3 )δ–93.54(s,2F); 13 C NMR (100MHz, CDCl 3 )δ189.2(t, J=31.1Hz), 137.8, 137.1, 135.4(t, J=22.8Hz), 134.5, 133.4, 132.5, 132.1, 131.3(t, J=4.0Hz), 130.3, 129.7, 129.7 ,128.3,126.9(t,J=8.3Hz),116.4(t,J=254.2Hz),21.2,21.0; HRMS(ESI)calcd for[C 20 h 18 f 2 OSNa (M+...
Embodiment 3
[0059] In a 50 mL dry reaction tube, add 4,4'-dibromodiphenyl sulfoxide (360.1 mg, 1.0 mmol), phenyl difluoroenol silyl ether (342.5 mg, 1.5 mmol), dichloromethane 10.0 mL , then dropwise added trifluoroacetic anhydride (315.0mg, 1.5mmol), stirred magnetically at -10°C for 12 hours, detected by TLC, the raw material disappeared, concentrated directly, and further purified by column chromatography to obtain the product, with a yield of 86%;
[0060] 2-(5-Bromo-2-((4-bromophenyl)thio)phenyl)-2,2-difluoro-1-acetophenone (3d)
[0061] 1 H NMR (400MHz, CDCl 3 )δ8.02–7.94(m,3H),7.66–7.57(m,1H),7.54(dd,J=8.4,2.2Hz,1H),7.51–7.43(m,2H),7.40–7.33(m, 2H), 7.17(d, J=8.4Hz, 1H), 7.00–6.90(m, 2H); 19 F NMR (376MHz, CDCl 3 )δ–93.64(s,2F); 13 C NMR (100MHz, CDCl 3)δ188.4(t, J=31.1Hz), 137.4(t, J=23.2Hz), 136.0, 134.5, 133.9, 133.2(t, J=3.8Hz), 132.8(t, J=2.5Hz), 132.3 , 131.7, 130.3–129.4(m), 128.6, 115.9(t, J=256.5Hz); HRMS(ESI)calcdfor[C 20 h 12 Br 2 f 2 OSNa (M+Na + )]:518.8836...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com