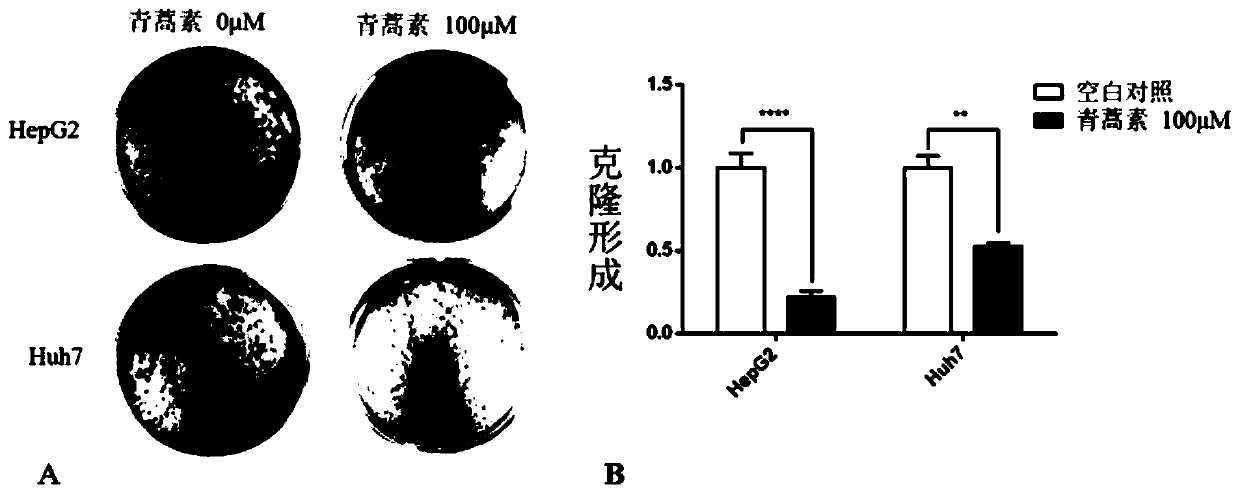
Application of artemisinin in preparation of drugs for resisting human liver cancer HepG2 and Huh7 cells
An artemisinin and cell technology, applied in antitumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of difficulty in early detection of HCC and low survival rate of HCC patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The artemisinin medicine is made into granules, and the preparation method is as follows:
[0054] 1) Take 50g of artemisinin, add water and decoct twice, the first time for 2 hours, the second time for 1 hour, combine the decoction, filter, and concentrate the filtrate to an appropriate amount (about 50mL);
[0055] 2) Add ethanol to make the alcohol content 60%, stir while adding, let it stand for precipitation, take the supernatant to recover ethanol, concentrate to a clear paste with a relative density of 1.30-1.33 (80°C) (about 1:4, That is, 1 part of clear ointment is equivalent to 4 parts of medicinal materials);
[0056] 3) Add 20g of a mixture of sugarcane powder and dextrin (sucrose:dextrin=3:1) and 10ml of 70% ethanol, mix to form soft powder, squeeze and sieve (12-14 mesh) to make granules;
[0057] 4) Dried at 60°C, granulated, packed in plastic bags equivalent to 10 g of artemisinin per bag, and sealed to obtain artemisinin granules.
[0058] Dosage meth...
Embodiment 2
[0061] The artemisinin medicine is made into capsules, and the preparation method is as follows:
[0062] 1) Take 25 g of artemisinin and pulverize it into a coarse powder, reflux with 95% ethanol for 1 hour, and filter; then reflux the dregs with 50% ethanol for 1.5 hours, filter, combine the filtrates, and recover ethanol;
[0063] 2) Decoct the dregs with water for 2 hours, filter, combine the decoction and filtrate, and concentrate until syrupy;
[0064] 3) Add 10g of starch and mix well, add an appropriate amount of starch slurry, mix to make soft powder, press through a 80-100 mesh sieve to granulate, dry at 60-70°C, and granulate;
[0065] 4) Add 0.15 g of magnesium stearate, mix well, and pack into capsules to obtain artemisinin capsules.
[0066] Administration method: Oral, 1 to 2 times a day, 1 to 2 capsules each time, suitable for consumption on an empty stomach. Those with sensitive stomachs, please take with meals.
[0067] Precautions: Keep out of reach of ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com