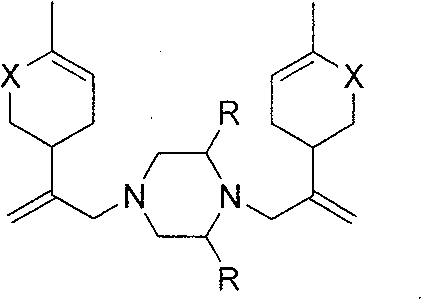
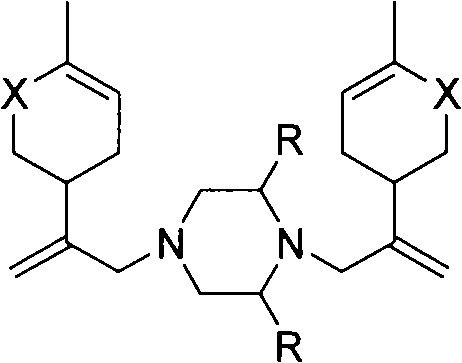
Piperazine derivant with antineoplastic activity
A kind of derivative, the technology of piperazine, applied in the field of piperazine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
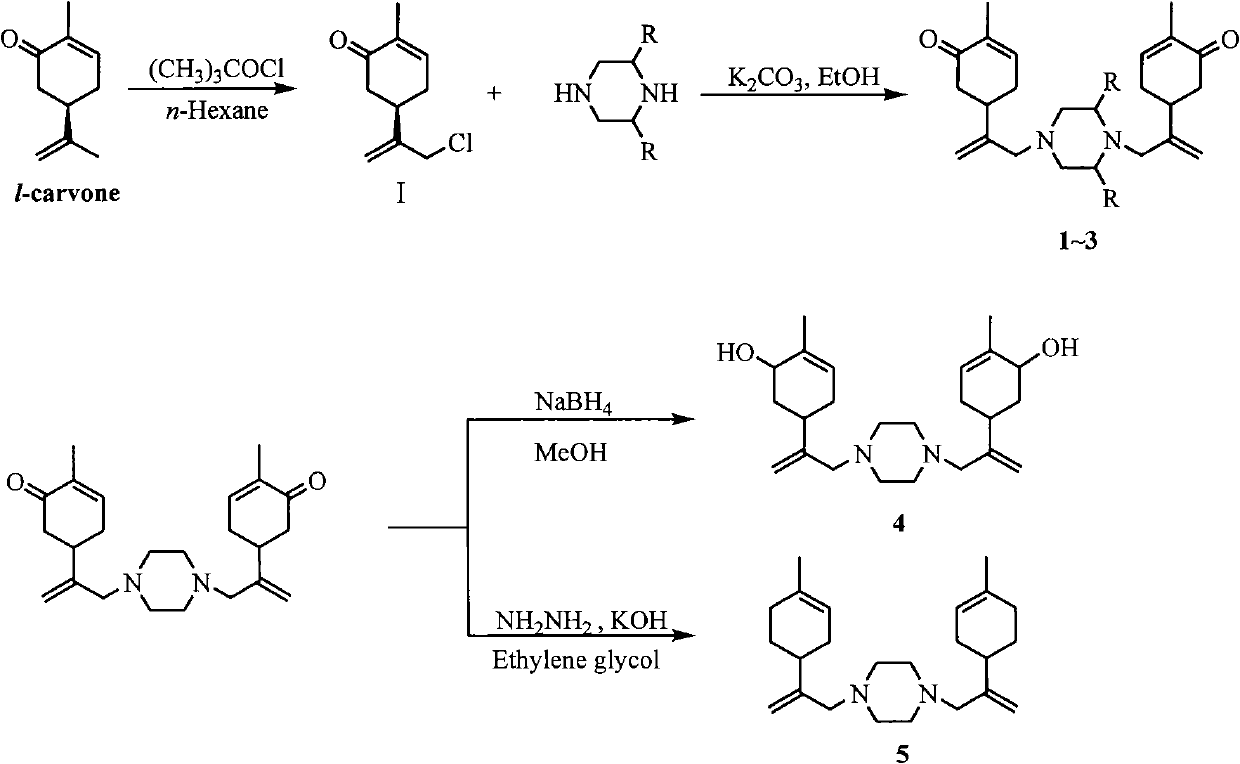
[0016] Prepare the chlorinated carvone intermediate by the existing mature method: dissolve 15.0 g (0.1 mol) of carvone in 300 mL of n-hexane, place in an ice bath at 0-5°C, and add 13.3 tert-butyl hypochlorite dropwise under stirring. g (0.11mol), the addition was completed and raised to room temperature, and continued to stir for 3h. The reaction solution was washed successively with saturated aqueous sodium sulfite solution and saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 10.7 g of a yellow oil with a yield of 72.7%. 1 H NMRδ(ppm): 1.76(s, 3H), 2.33-2.40(m, 2H), 2.49-2.66(m, 2H), 2.94(m, 1H), 4.06(s, 2H), 5.03(s, 1H ), 5.23(s, 1H), 6.73(m, 1H).
Embodiment 2
[0018] Synthesis of N,N-bis[2-(4-methyl-5-oxocyclohex-3-ene)allyl]piperazine hydrochloride (1)
[0019] Dissolve 16.2mmol of chlorocarvone intermediate, 8.9mmol of potassium carbonate, and 8.1mmol of piperazine in 15mL of ethanol, and heat to reflux for 8h. Ethanol was evaporated, the residue was dissolved in 30mL dichloromethane, washed with 1N hydrochloric acid solution (30mL×3), the acid solution was combined, neutralized with anhydrous sodium carbonate to pH9.0~10.0, extracted with dichloromethane (30mL×5 ), the organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a yellow oil, which was dissolved in hydrochloric acid ethanol and allowed to stand for crystallization to obtain a white crystalline solid. Product yield: 67.9%, mp: 195-197°C. 1 H-NMRδ(ppm): 1.77(s, 6H), 2.20-2.67(m, 16H), 2.85(m, 2H), 2.92(s, 4H), 4.92(s, 2H), 5.01(s, 2H) , 6.74 (m, 2H); MS (EI) m / z: 382 (M + , 100)...
Embodiment 3
[0021] Synthesis of 2-methyl-N,N-bis[2-(4-methyl-5-oxocyclohex-3-ene)allyl]piperazine hydrochloride (2)
[0022] Reaction process is with embodiment 2. Product yield: 77.4%, mp: 190-192°C. 1 H NMRδ(ppm): 1.24(m, 3H), 1.79(s, 6H), 2.30-2.49(m, 15H), 2.84(m, 2H), 2.92(s, 4H), 4.91(s, 2H), 5.02(s, 2H), 6.75(m, 2H); MS(EI) m / z: 410(M + , 5), 192(100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com