Analytical kit for detecting four free fatty acids in human blood spots through high performance liquid chromatography-tandem mass spectrometry
A high-performance liquid chromatography and free fatty acid technology, applied in the field of analysis and detection, can solve the problems of low detection sensitivity and complicated operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] The analysis kits are listed in Table 5.
[0106] Table 5 Preparation of Analysis Kit Components
[0107]
[0108] The preparation method of the above-mentioned kit is as follows:
[0109] (1) Standard product:
[0110] The preparation of the standard substance with the concentration of J1: take 160 μL of 50 mM α-octadecatrienoic acid, 8 μL of 100 mM eicosapentaenoic acid, 20 μL of 100 mM ω3-docosapentaenoic acid, and 160 μL of 50 mM docosahexaenoic acid, Add 1652 μL of methanol respectively to prepare 4 μM α-octadecatrienoic acid, 0.4 μM eicosapentaenoic acid, 1 μM ω3-docosapentaenoic acid and 4 μM docosahexaenoic acid; Then take 150 μL each of 4 μM α-octadecatrienoic acid, 0.4 μM eicosapentaenoic acid, 1 μM ω3-docosapentaenoic acid and 4 μM docosahexaenoic acid, and use artificial rabbit The blood volume was adjusted to 3000 μL, and the obtained concentration was J1 standard;
[0111]Preparation of standard substance with concentration J2: Take 500 μL each of α...
Embodiment 2
[0129] 1. Pretreatment
[0130] Test sample processing: (1) Extraction: Take a dried blood spot sample with a diameter of 6mm in a 2mL centrifuge tube, add 200μL of the extraction solution containing the internal standard, and then vortex and shake at 2500rpm for 30min, take 150μL of the above mixed solution and use a nitrogen meter Blow dry; (2) Derivatization: add 200 μL derivatization reagent 1 (10vt% acetyl chloride in dichloromethane solution), derivatize with vortex shaking at 500rpm for 30min at room temperature, and dry; then add 200 μL derivatization reagent 2 (1vt% Acetonitrile solution of picolylamine), derivatized by vortexing at 500rpm for 5min at room temperature, and dried; (3) Reconstitution: add 400μL reconstitution solution (saturated monohydric alcohol), and vortex and mix at 2500rpm for 5min at room temperature .
[0131] Standard product: the processing method is the same as that of the test sample, and will not be repeated here;
[0132] Quality control...
Embodiment 3
[0162] Example 3: Performance Verification
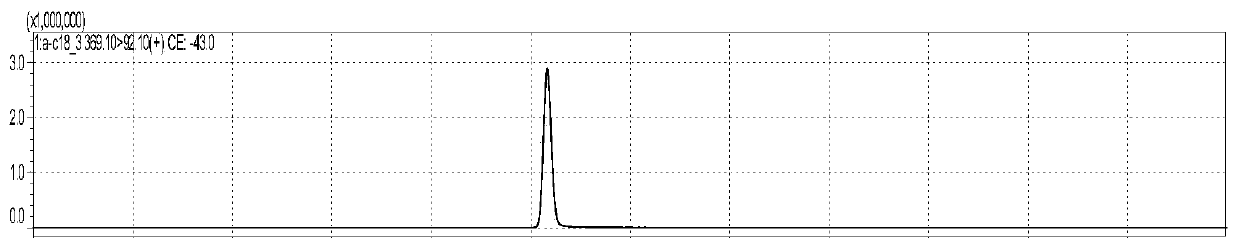
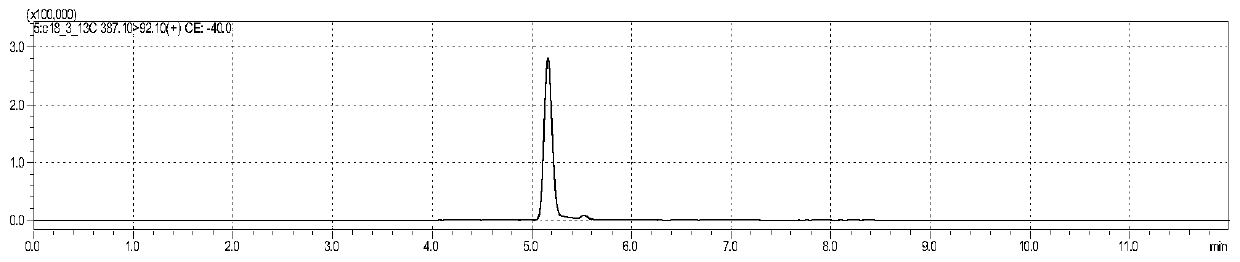
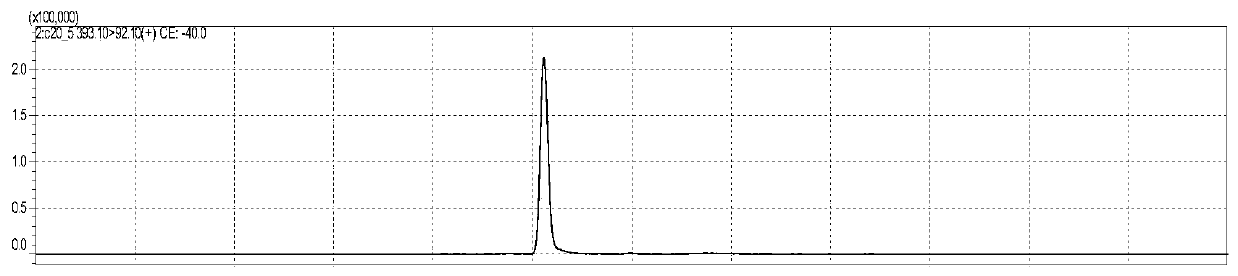
[0163] figure 1 is the chromatogram of alpha-octadecatrienoic acid standard substance, figure 2 Octadecatrienoic acid- 13 C18 isotope internal standard chromatogram, image 3 is the chromatogram of eicosapentaenoic acid standard substance, Figure 4 is eicosapentaenoic acid-d5 isotope internal standard chromatogram, Figure 5 is the chromatogram of ω3-docosapentaenoic acid standard substance, Figure 6 It is the internal standard chromatogram of docosahexaenoic acid-d5 isotope (which is the internal standard of ω3-docosapentaenoic acid). Figure 7 is the docosahexaenoic acid standard chromatogram, Figure 8 Docosahexaenoic acid-d5 isotope internal standard chromatogram (internal standard for two compounds of docosahexaenoic acid). , the peak positions of each substance are 5.033min, 5.052min, 5.049min, 5.058min, 6.626min, 5.939min, 6.094min and 5.939min, although these 8 substances are not completely separated, but for LC-MS / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com