Method for preparing ethanol
A technology of ethanol and acetic acid, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of incomplete vaporization of acetic acid, large equipment investment, and large corrosiveness, so as to reduce energy consumption and equipment investment , low conversion rate requirements, and the effect of reducing corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Acetic acid catalytic hydrogenation catalyst (Pt-Sn-Cu-Zn-Mg / SiO 2 Preparation of catalyst): Weigh 10 grams of strip high-purity SiO 2 Porous carrier with a diameter of 3 mm, a length of 5 mm, a pore volume of 1 ml / g, and a BET specific surface area of 250 cm 2 / g, the water absorption rate of the carrier was measured to be 1.2 ml / g, and the ethanol saturation adsorption rate was 1.4 ml / g. Weigh 0.038 g SnCl 2 ·2H 2 O, which was dissolved in 14 ml of ethanol to form SnCl 2 immersion liquid. The above SnCl 2 The dipping solution was slowly added dropwise to the SiO 2 The porous carrier was dried at 110° C. for 5 hours, then heated to 500° C. and calcined for 5 hours to obtain a SnO-loaded SiO2 porous carrier. 0.152 g of copper nitrate, 0.045 g of zinc nitrate and 0.105 g of magnesium nitrate were weighed and dissolved in 12 ml of deionized water to prepare a co-dipping solution. The above co-dipping solution was slowly added dropwise to the above-mentioned SnO-...
Embodiment 2
[0057] Pt-Sn-Cu-Zn-Mg / SiO 2 Preparation of catalyst: same as Example 1.
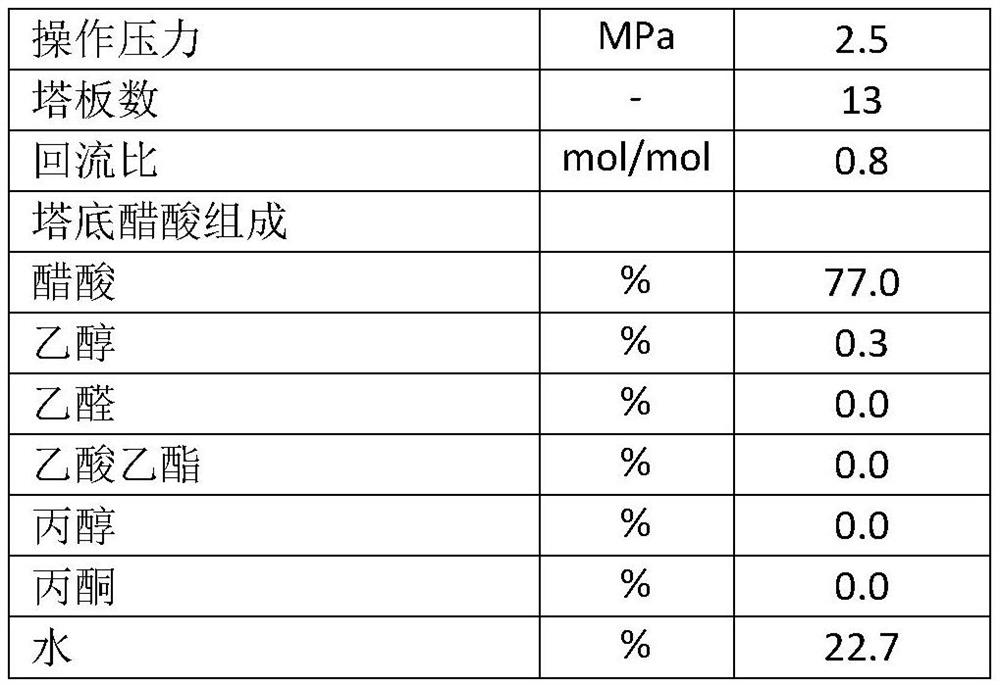
[0058] like figure 1 As shown, the acetic acid raw material 101 from outside the boundary area is boosted to the reaction pressure by the acetic acid feed pump 1, then enters the acetic acid preheater 11 to be preheated to a certain temperature, and then enters the vaporizer 2; the fresh hydrogen 103 from outside the boundary area and The circulating hydrogen 114 from the circulating hydrogen compressor 7 is mixed in a certain proportion, and the mixed hydrogen 104 is heated to a certain temperature by the hydrogen preheater 12, and enters the vaporizer 2 together with the acetic acid 102 from the acetic acid preheater 11; The nozzle on the vaporizer 2 forms a spray and enters the vaporizer 2 to achieve the purpose of vaporizing acetic acid; the acetic acid and hydrogen mixture stream 105 is heated to the feed temperature by the mixed raw material heater 13, and enters the hydrogenation reactor 3, where...
Embodiment 3
[0072] Preparation of Pt-Sn-Cu-Zn-Mg / AC catalyst: According to the method of Example 1, the difference is that the SiO 2 The porous carrier becomes coconut shell activated carbon (AC, its saturated water absorption rate is 13 ml / g, and the ethanol saturated adsorption rate is 15 ml / g), SnCl 2 ·2H 2 The amount of O was changed to 0.570 g, the amount of copper nitrate was changed to 3.422 g, the amount of zinc nitrate was changed to 2.275 g, the amount of magnesium nitrate was changed to 5.274 g, and the amount of [Pt(NH) 3 ) 4 ](NO 3 ) 2 Except that the dosage is changed to 0.180 g, the preparation process of Example 1 is repeated to obtain an activated carbon carrier supporting the respective oxides of Pt, Sn, Cu, Zn and Mg, that is, a catalyst precursor (a catalyst that is not activated by reduction), and the activated carbon carrier after reduction activation is obtained. The chemical composition is Pt:carrier weight ratio / Pt:Sn:Cu:Zn:Mg mass ratio of 9:100 / 0.9:3:9:5:5. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com