A kind of metal pillar modified layered manganese birnessite and its preparation and application
A modified layer and metal pillar technology, applied in the field of metal pillar modified layered manganese Birnessite and its preparation, can solve the problems of easy poisoning and deactivation, increase of industrial cost, etc., achieve strong chlorine poisoning resistance, energy saving, The effect of easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The object of the present invention is to provide a kind of preparation method of metal pillar modified layered manganese Birnessite, described preparation method comprises the following steps:
[0041] Step 1: Prepare a layered manganese precursor, and the product is denoted as K-Bir;
[0042] Step 2: carry out hydrogen ion exchange, and the product is denoted as H-Bir;
[0043] Step 3: Perform pillar replacement, and the product is denoted as A-Bir;
[0044] Step 4: introduce metal ion reaction, the product is recorded as AM-Bir;
[0045] Step 5: Roasting, the product is recorded as M-Bir.
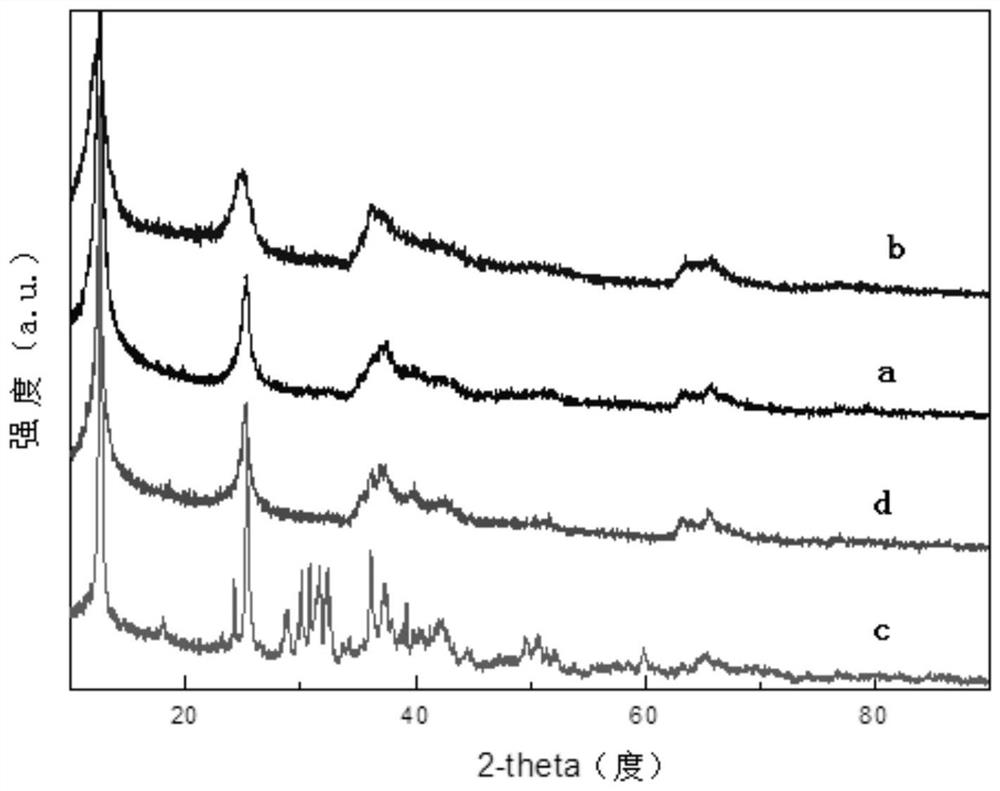
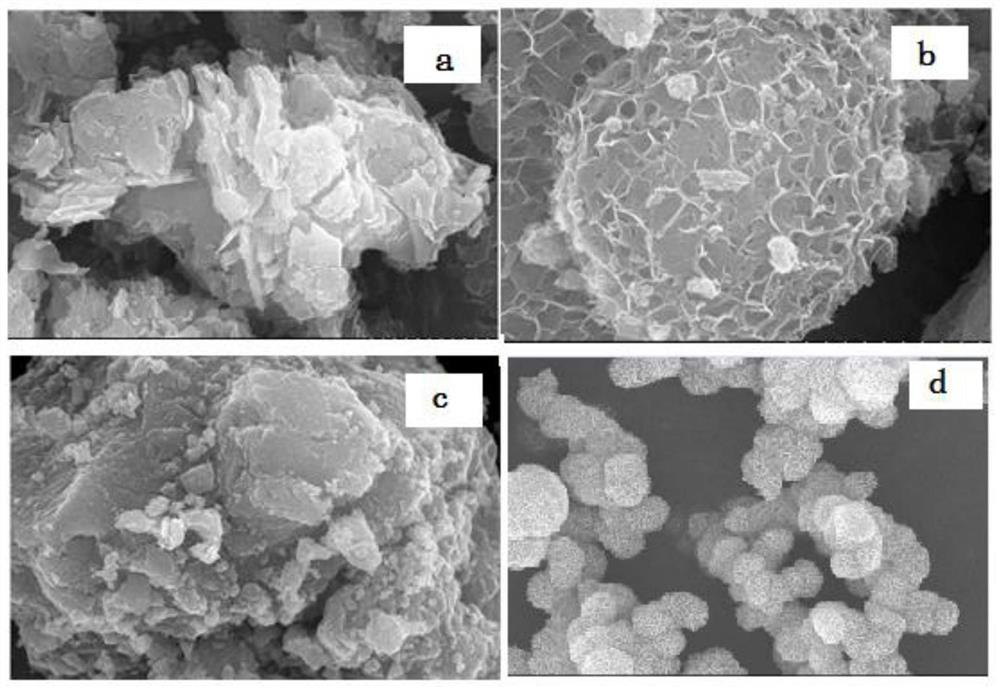
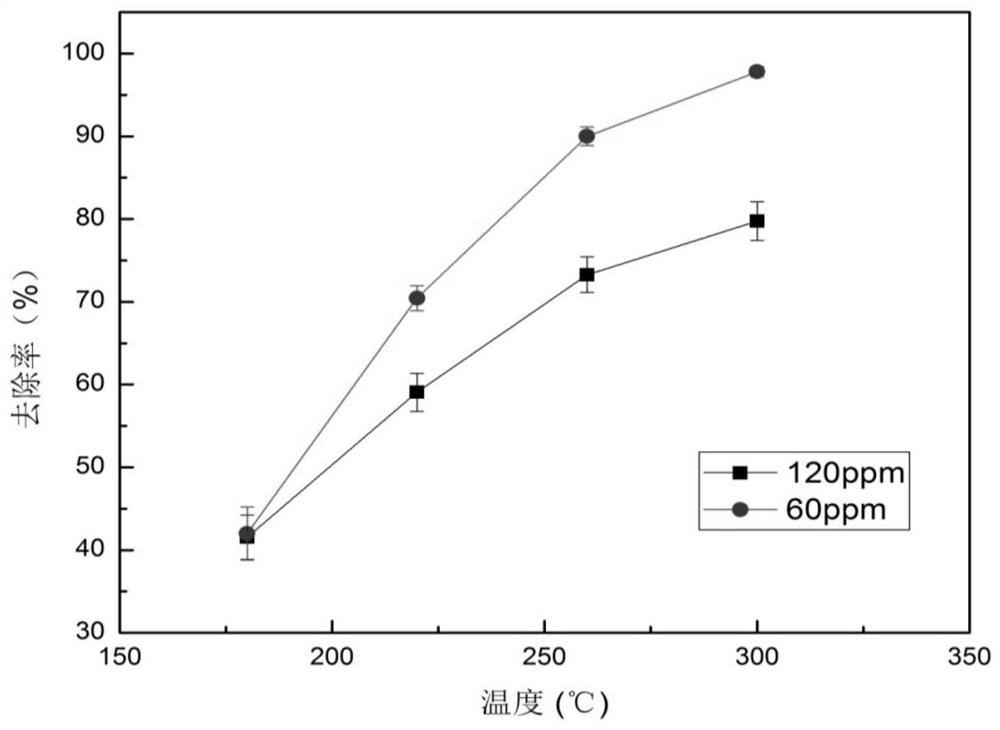
[0046] MnO with layered structure 2 The crystalline compounds are collectively called δ-MnO 2 , the space group belongs to C2 / m, it is a monoclinic crystal system, and the [MnO 6 ] Formed by a common edge. Among them, some Mn 3+ ion substitution Mn 4+ The position makes the manganese and oxygen negatively charged, while metal cations and organic macromolecules can enter th...
Embodiment 1
[0123] Add 100ml deionized water to the hydrothermal kettle, then add 1.58g KMnO 4 Add it into a hydrothermal kettle, and stir in deionized water for 30 minutes, then add 2ml of 0.15mol / L ammonia water; then react the added raw materials in a hydrothermal kettle at 160°C for 12h, and the reaction ends;
[0124] Filter the solid-liquid mixture of the reaction solution in the hydrothermal kettle, and wash the filter cake with deionized water until the pH value of the filtrate is 7 to obtain the filter cake; then dry the filter cake at 80°C for 12 hours to obtain K-Bir;
[0125] The resulting K-Bir was placed in 1M HNO 3 In the solution, heat and stir at 40°C for 24 hours to carry out hydrogen ion exchange to prepare H-Bir;
[0126] Put the obtained H-Bir in 0.5 mol / L n-octylamine-ethanol solution, the molar ratio of n-octylamine to manganese is 5:1, heat and stir at 45°C for 24h to carry out pillar replacement, suction filtration, Dry the filter cake at 80°C for 12 hours to ob...
Embodiment 2
[0130] Add 100ml deionized water to the hydrothermal kettle, then add 1.58g KMnO 4 Add it into a hydrothermal kettle, stir in deionized water for 30 minutes, then add 2ml of 0.15mol / L citric acid; then react the added raw materials in a hydrothermal kettle at 160°C for 12h, and the reaction is complete;
[0131] Filter the solid-liquid mixture of the reaction solution in the hydrothermal kettle, and wash the filter cake with deionized water until the pH value of the filtrate is 7 to obtain the filter cake; then dry the filter cake at 80°C for 12 hours to obtain K-Bir;
[0132] The resulting K-Bir was placed in 1M HNO 3 In the solution, heat and stir at 40°C for 24 hours to carry out hydrogen ion exchange to prepare H-Bir;
[0133] Put the obtained H-Bir in 0.5mol / L n-octylamine ethanol solution, the molar ratio of n-octylamine to manganese is 5:1, heat and stir at 45°C for 24h to carry out pillar replacement, suction filter, and The filter cake was dried at 80°C for 12 hours...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com